Benralizumab Overview
Introduction of Benralizumab
Benralizumab is a humanized monoclonal antibody which binds to the α subunit of the Interleukin-5 receptor (IL-5R), and indicated for the add-on maintenance treatment of patients with severe asthma aged 12 years and older, and with an eosinophilic phenotype. As Interleukin-5(IL-5) is implicated in disease states that are mediated by eosinophils, benralizumab is an attractive option for use in the treatment of asthma. Benralizumab has been approved for marketing by FDA on November 14, 2017.
Mechanism of Action of Benralizumab
As eosinophils are the dominating effector cells in asthma, inhibition of eosinophilia, theoretically would result in a decreased airway injury, mucus hypersecretion and bronchial hyper-responsiveness. IL-5 induces an inflammatory response by interacting with eosinophils through targeting the Interleukin-5 receptor (IL-5R) expressed in eosinophils, basophils and some mast cells. IL-5R is a heterodimer, composed of a monomeric α and a dimeric β chain. The α chain specifically binds with IL-5 with low affinity, however, the ligand binding of IL-5 and IL-5Rα is followed by hetero-oligomerization of α and β subunits that leads to further proximity of β subunit intracellular domains producing a high-affinity phenotype. Benralizumab, binds with high affinity to of IL-5Rα, which in turn blocks the ligand binding of IL-5 as well as other putative ligands (such as IL-3, IL-4, IL-13 and GM-CSF), thereby no hetero-oligomerization of α and β subunits takes place and thus no signal transduction occurs (Figure 1).
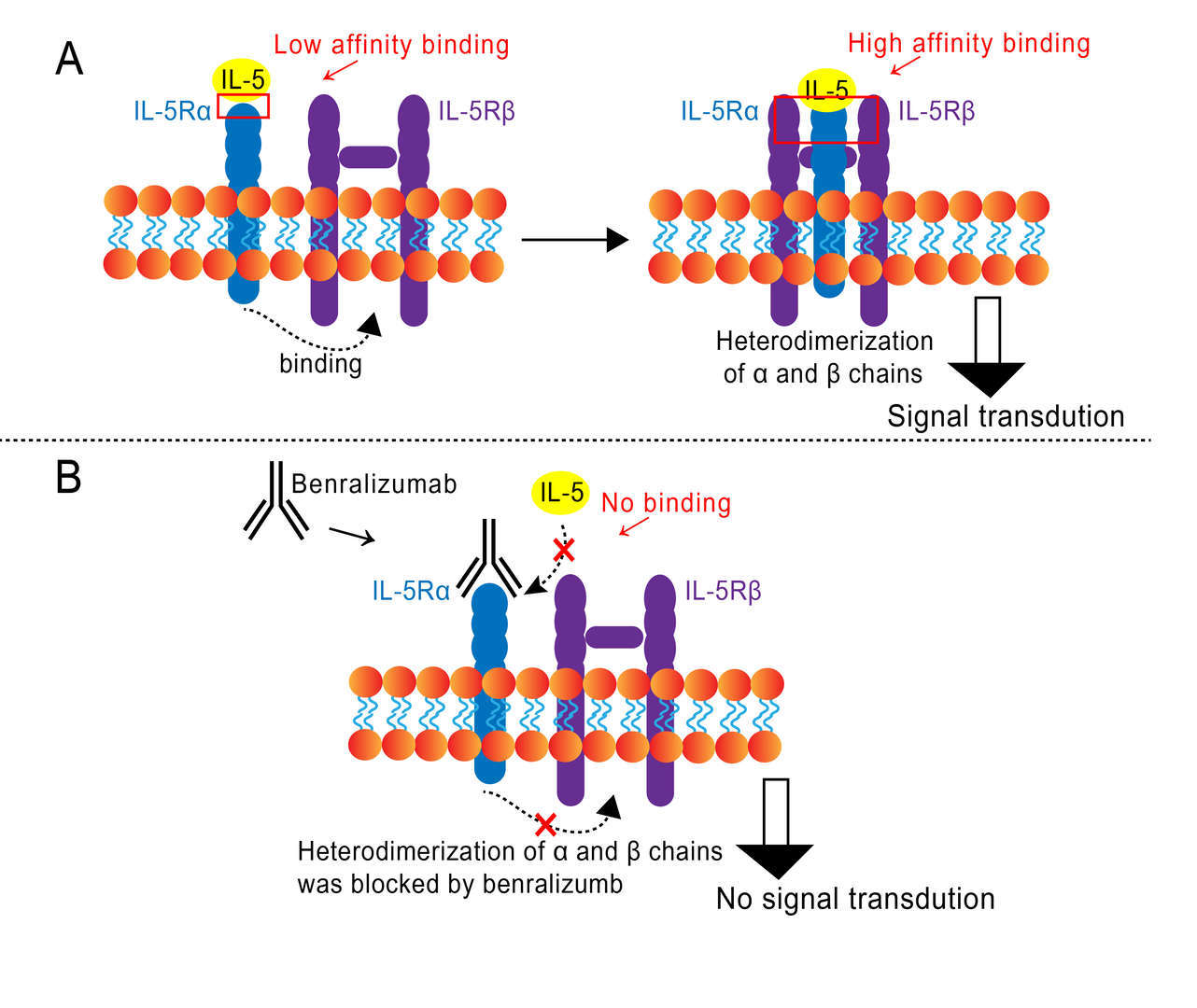 Figure 1 Mechanism of Action of Benralizumab
Figure 1 Mechanism of Action of Benralizumab
Clinical Projects of Benralizumab *
| NCT ID | Status | Conditions | Lead Sponsor | Update Time |
| NCT03473977 | Recruiting | Eosinophilic Gastritis or Gastroenteritis | Children's Hospital Medical Center, Cincinnati | March 22, 2018 |
| NCT02869438 | Active, not recruiting | Asthma | AstraZeneca | August 17, 2016 |
| NCT02258542 | Active, not recruiting | Asthma | AstraZeneca | October 7, 2014 |
| NCT03470311 | Not yet recruiting | Severe Prednisone Dependent Eosinophilic Asthma | McMaster University | March 19, 2018 |
| NCT03401229 | Recruiting | Nasal Polyposis | AstraZeneca | January 17, 2018 |
| NCT02821416 | Recruiting | Asthma | AstraZeneca | July 1, 2016 |
| NCT03186209 | Recruiting | Asthma | AstraZeneca | June 14, 2017 |
| NCT03170271 | Recruiting | Asthma | AstraZeneca | May 31, 2017 |
| NCT03327701 | Not yet recruiting | Asthma, Exercise-Induced | Louis-Philippe Boulet | October 31, 2017 |
| NCT02808819 | Active, not recruiting | Asthma | AstraZeneca | June 22, 2016 |
| NCT03450083 | Recruiting | Chronic Rhinosinusitis (Diagnosis), Nasal Polyps, Eosinophilia | Johns Hopkins University | March 1, 2018 |
| NCT03010436 | Recruiting | Asthma | National Jewish Health | January 5, 2017 |
| NCT02130882 | Active, not recruiting | Hypereosinophilic Syndrome | National Institute of Allergy and Infectious Diseases (NIAID) | May 6, 2014 |
| NCT03183024 | Recruiting | Chronic Idiopathic Urticaria | Jonathan A. Bernstein, MD | June 9, 2017 |
Approved Drugs of Benralizumab **
| INN (trade name) | Therapeutic area | Dose | Strength | Route | Company | Marketing start | Market |
| Fasenra® | Asthma | Injection, solution | 30 mg/mL | Subcutaneous | AstraZeneca Pty Ltd | April 2, 2018 |

|
| Fasenra® | Asthma | Injection, solution | 30 mg/mL | Subcutaneous | AstraZeneca AB | November 14, 2017 |

|
| Fasenra® | Asthma | Injection, solution | 30 mg/mL | Subcutaneous | Astra Zeneca | April, 2018 |

|
| Fasenra® | Asthma | Injection, solution | 30 mg/mL | Subcutaneous | Astra Zeneca | November 14, 2017 |

|
Resources
* The table was excerpted from the following website
https://clinicaltrials.gov/ct2/results?cond=&term=Benralizumab&cntry=&state=&city=&dist=
** Information presented in the table were collected from the following websites:
https://search.tga.gov.au/s/search.html?collection=tga-websites-web&query=Benralizumab&op=Search
http://www.ema.europa.eu/ema/index.jsp?curl=search.jsp&q=Benralizumab+&btnG=Search&mid=
https://www.kegg.jp/medicus-bin/search_drug?search_keyword=Benralizumab
https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=BasicSearch.process
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

