Concizumab Overview
Introduction of Concizumab
Concizumab (mAb 2021) is a high-affinity, humanized, monoclonal IgG4 antibody (mAb). It was directed against the Kunitz-2 domain of human tissue factor (TF) pathway inhibitor (TFPI), designed to target and selectively block the factor X (FX) a (FXa) binding site of TFPI. As a mAb, concizumab has the advantage of being administered subcutaneously, and exhibits good solubility and stability, allowing administration as a liquid formulation via a ready- and easy-to-use portable pen device.
Mechanism of Action of Concizumab
Severe haemophilia A and B are characterized by recurrent bleeding into joints, muscles and soft tissues. Primary prophylaxis with infusions of coagulation factor VIII (FVIII; haemophilia A) or factor IX (FIX; haemophilia B) is the gold standard of care for haemophilia patients today, with the aim of preventing bleeds, slowing the progression of joint destruction and preserving musculoskeletal function. However, there is growing interest in the development of novel, non-replacement therapies with alternative mechanisms of action and modes of administration that may improve compliance. One potential target for alternative treatment strategies is tissue factor pathway inhibitor (TFPI), the primary inhibitor of the initiation of coagulation. It binds and inhibits factor VIIa (FVIIa) in complex with tissue factor (TF), and factor Xa (FXa), with the overall effect of reduced thrombin generation and decreased blood coagulation. Concizumab abolishes TFPI inhibition of the TF pathway resulting in increased FXa production, thus allowing sufficient thrombin generation despite factor VIII (FVIII) or factor IX (FIX) deficiency in people with hemophilia A or B with or without inhibitors. Non-clinical studies have shown that concizumab binds to TFPI with high affinity and abrogates TFPI inhibition of TF-factor VIIa (FVIIa)-mediated FX activation and FXa activity in vitro. Furthermore, concizumab has been shown to promote TF-induced thrombin generation by neutralizing TFPI in FVIII-deficient plasma. Inhibition of TFPI also correlated with reduced blood loss and bleeding time in a hemophilia rabbit model.
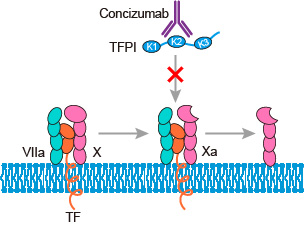 Fig.1 Mechanism of action of concizumab
Fig.1 Mechanism of action of concizumab
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.



