Daclizumab Overview
Introduction of Daclizumab
Daclizumab, also known as Zinbryta, BIIB019 and Zenepax, is a humanized monoclonal antibody that targets the IL-2 receptor α-chain (IL-2Rα, also known as CD25) on the surface of activated T-cells. CD25 is predominantly expressed on these activated T-cells, and its overexpression has been implicated in various autoimmune diseases and certain types of cancer, such as adult T-cell leukemia/lymphoma, where it plays a crucial role in various immune responses and the proliferation of malignant cells. Initially developed by PDL BioPharma, the antibody was humanized by grafting murine antigen-binding regions onto a human IgG1 framework, dramatically reducing its immunogenicity and enhancing compatibility with patients. Daclizumab, applied with cyclosporine and corticosteroids, was approved by the U.S. Food and Drug Administration in 1997 to prevent acute rejection of kidney transplants, though it was later withdrawn from the market in March 2018 following reports of autoimmune encephalitis in Europe. Apart from its application in transplant treatment, daclizumab has also shown potential in treating multiple sclerosis (MS) and other autoimmune conditions. One study that compared Zinbryta to Avonex (interferon beta 1a) in 1841 people suffered with MS over two years demonstrated that Zinbryta decreased the relapse rates in one year by 46% and reduced the quantity of lesions observed on MRI by 54%.
Biological and Chemical Properties of Daclizumab
Protein Structure
 Figure 1. The Structure of Human CD25 (UniProt)1,2
Figure 1. The Structure of Human CD25 (UniProt)1,2
Protein Chemical Formula
C6332H9808N1678O1989S42
Protein Average Weight
The average molecular weight of daclizumab is approximately 142612.1 Da.
The Mechanism of Daclizumab Action
IL-2 Signaling Pathway
To understand the mechanism of action, it is essential to first grasp the IL-2 signaling pathway. IL-2 is a cytokine produced by activated T-cells that plays a pivotal role in the proliferation, differentiation, and survival of these immune cells. The IL-2 receptor is composed of three subunits: IL-2Rα (CD25), IL-2Rβ (CD122), and the common gamma chain (γc or CD132). CD25 presents the highest affinity for IL-2, effectively acting as a sink for IL-2 on activated T-cells. Upon binding IL-2, the receptor undergoes a conformational change, triggering downstream signaling cascades, predominantly through the JAK-STAT pathway.
Mechanistic Insights into Daclizumab
Daclizumab exerts its immunomodulatory effects by binding with high specificity to the IL-2Rα (CD25) subunit, inhibiting the interaction between IL-2 and its high-affinity receptor. This inhibition prevents IL-2 dependent proliferation of activated T-cells, thereby curbing the expansion of both effector T-cells and regulatory T-cells (Tregs). This rebalancing of the immune system is particularly useful in conditions such as autoimmunity and transplant rejection, where excessive T-cell proliferation is detrimental.
Inhibition of T-cell Proliferation
The proliferation of T-cells is a hallmark of both acute and chronic immune responses. Daclizumab disrupts this process by targeting the IL-2Rα subunit, thereby blocking the critical proliferative signals required for T-cell clonal expansion. This action is beneficial in managing conditions like autoimmunity and transplant rejection, where controlling T-cell proliferation is crucial.
Modulation of Natural Killer (NK) Cells
Recent studies have shown that daclizumab also impacts NK cells, a subset of lymphocytes involved in immune surveillance and cytotoxicity. Daclizumab increases the number of CD56bright NK cells, which are known for their immunoregulatory functions. These NK cells can modulate immune responses by secreting anti-inflammatory cytokines such as IL-10, contributing to the overall therapeutic effects of daclizumab.
The following Figure 2 demonstrates the known and proposed mechanisms of action of daclizumab. Figure 2. (A) shows that: daclizumab binds to CD25, inhibiting its interaction with CD132 and CD122. This binding prevents CD25 from trans-presenting IL-2 from mature dendritic cells (mDC) to T cells, leading to the death of both effector T cells and regulatory T cells (Tregs). Figure 2 (B) indicates that CD25 inhibition by daclizumab increases serum IL-2 levels, which allows activation of natural killer (NK) cells via the intermediate-affinity receptors CD132 and CD122. This enhances the cytotoxicity and survival of CD56bright NK cells. The dotted lines in the figure represent the sequence of events following IL-2 binding to its receptor.
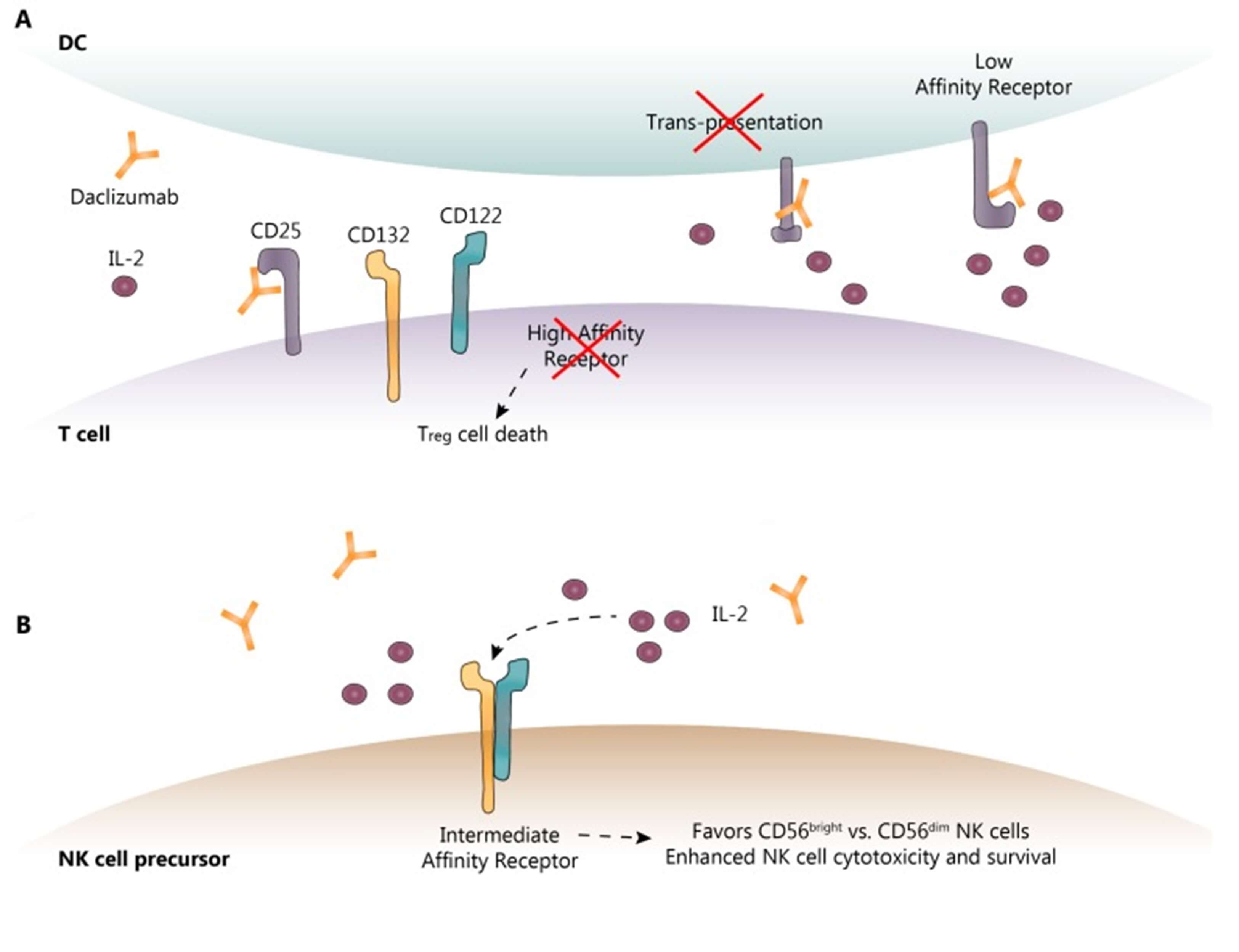 Figure 2. The Known and Proposed Mechanisms of Daclizumab Action3,4
Figure 2. The Known and Proposed Mechanisms of Daclizumab Action3,4
The Clinical Applications of Daclizumab
Kidney Transplantation
Daclizumab was initially developed to reduce the incidence of acute organ rejection following kidney transplantation. Its efficacy in this area has been demonstrated in several Phase III clinical trials. When used as part of an induction regimen, daclizumab significantly reduced the risk of rejection episodes during the critical post-transplant period without compromising overall patient and graft survival.
Multiple Sclerosis
The therapeutic scope of daclizumab has expanded significantly with its application in treating relapsing forms of multiple sclerosis (MS), a debilitating autoimmune disorder characterized by the demyelination of central nervous system axons. Daclizumab, through its dual action on T-cell and NK-cell modulation, has demonstrated substantial efficacy in reducing relapse rates and slowing disease progression.
In various clinical trials, including the pivotal DECIDE study, daclizumab showed a significant reduction in the annualized relapse rate compared to interferon beta-1a, a standard treatment for MS. While generally well-tolerated, daclizumab is associated with potential adverse effects, such as liver toxicity and serious infections, requiring regular monitoring.
Other Autoimmune Disorders
Given the central role of IL-2 in immune regulation, daclizumab's application has been explored in various autoimmune diseases beyond MS. Although initial results have been promising, more extensive clinical trials are required to validate its efficacy and safety in these broader contexts.
Clinical Projects of Daclizumab*
| NCT ID | Study Title | Study Status | Conditions | Start Date |
| NCT01929746 | Daclizumab Japanese PK Study | Completed | Healthy | 2013-10 |
| NCT00040248 | Daclizumab to Treat Wegener's Granulomatosis | Completed | Wegener's Granulomatosis | 2002-06 |
| NCT00080431 | Daclizumab to Treat HIV-Infected Patients | Completed | HIV Infections | 2004-03-26 |
* The table above is excerpted from https://clinicaltrials.gov/search?cond=%20Daclizumab .
Approved Drugs of Daclizumab**
| INN (trade name) | Therapeutic area | Dosage | Strength | Route | Action Date |
| ZINBRYTA | multiple sclerosis (MS) | 150 milligrams once monthly | 150MG/ML | INJECTION | 05/27/2016 |
** Information in this table is sourced from the following website: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=BasicSearch.process
What We Provide
Anti-Human CD25 Recombinant Antibody (Daclizumab)
We provide high-quality Daclizumab for use in ELISA, Neut, IF, IP, FC, FuncS, and MRI. This product is intended for laboratory research use only and is not for diagnostic, therapeutic, or any in vivo human use.
- Immunogen
- The details of the immunogen for this antibody are not available.
- Host Species
- Mouse
- Derivation
- Humanized (from mouse)
- Type
- IgG1 - kappa
- Specificity
- Tested positive against native human antigen
- Species Reactivity
- Human
- Applications
- ELISA, Neut, IF, IP, FC, FuncS, MRI
- Trade name
- daclizumab
- CAS
- 152923-56-3
- Generic Name
- Daclizumab
- Biological Half-Life
- 20 days (11–38 days)
- ATC Code
- L04AC01
- DrugBank
- DB00111
- UNII
- CUJ2MVI71Y
- ChEMBL
- CHEMBL1201605
- MW
- 142,612.1 g/mol
- Related Disease
- Multiple sclerosis (MS)
- UniProt Database (https://www.uniprot.org/uniprotkb/P01589/entry)
- The image was retrieved from UniProt Database and used under [CC BY 4.0]. It was not modified and the titles was " The Structure of Human CD25 (UniProt)".
- Cohan, Stanley L et al. "Daclizumab: Mechanisms of Action, Therapeutic Efficacy, Adverse Events and Its Uncovering the Potential Role of Innate Immune System Recruitment as a Treatment Strategy for Relapsing Multiple Sclerosis." Biomedicines vol. 7,1 18. 11 Mar. 2019.
- Images retrieved from Figure 2 "Daclizumab: Mechanisms of Action, Therapeutic Efficacy, Adverse Events and Its Uncovering the Potential Role of Innate Immune System Recruitment as a Treatment Strategy for Relapsing Multiple Sclerosis." Cohan, Stanley L, 2019, used under [CC BY 4.0] (https://creativecommons.org/licenses/by/4.0/). The image was not modified compared to the original figure, and title was changed to " The Known and Proposed Mechanisms of Daclizumab Action ".
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.



