Daratumumab Overview
Introduction of Daratumumab
Daratumumab, a novel therapeutic human IgG1κ monoclonal antibody with high-affinity against the unique CD38 epitope, is an anti-cancer drug used in combination with other antineoplastic agents in the therapy of multiple myeloma. It was generated by the immunization of transgenic mice possessing the human immunoglobulin gene with recombinant CD38 protein and NIH 3T3 (expressing human CD38) cells until CD38-specific serum titer development. Daratumumab was granted approval by the U.S. Food and Drug Administration for treatment of multiple myeloma in patients who had received at least three prior therapies. And it was also conditionally approved for treatment of multiple myeloma in Europe, Canada, Japan, and Australia.
Mechanism of Action of Daratumumab
The therapeutic effects of daratumumab in multiple myeloma are mainly based on the mechanism that daratumumab binds to CD38 expressed on the surface of multiple myeloma cells to induce rapid cell death of multiple myeloma cells through complement-dependent cytotoxicity (CDC), antibody-dependent cell-mediated cytotoxicity (ADCC), antibody-dependent cellular phagocytosis (ADCP), apoptosis upon secondary crosslinking, and immunomodulatory effects via a decrease in immune suppressive cells (Fig. 1).
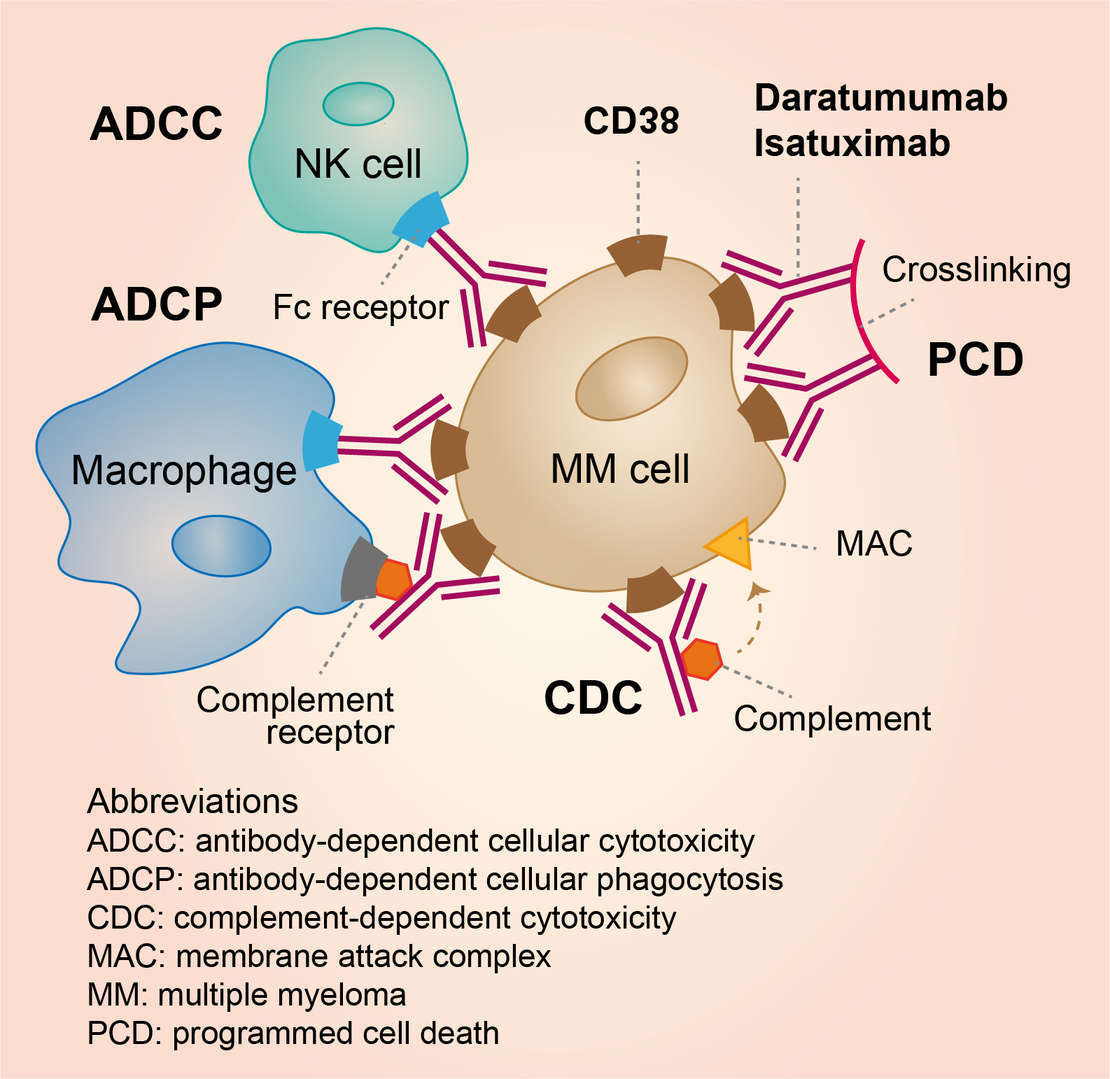 Fig.1 Mechanism of Action of Daratumumab
Fig.1 Mechanism of Action of Daratumumab
Clinical Projects of Daratumumab*
| NCT ID | Status | Conditions | Lead Sponsor | Update Time |
| NCT01084252 | Recruiting | Hematological Malignancy | Sanofi | March 10, 2010 |
| NCT01592370 | Active, not recruiting | Non-Hodgkin's Lymphoma, Hodgkin Lymphoma, Multiple Myeloma | Bristol-Myers Squibb | May 7, 2012 |
| NCT01615029 | Active, not recruiting | Multiple Myeloma | Janssen Research & Development, LLC | June 8, 2012 |
| NCT01946477 | Active, not recruiting | Multiple Myeloma | Celgene | September 19, 2013 |
| NCT01998971 | Active, not recruiting | Multiple Myeloma | Janssen Research & Development, LLC | December 3, 2013 |
| NCT02076009 | Active, not recruiting | Multiple Myeloma | Janssen Research & Development, LLC | March 3, 2014 |
| NCT02136134 | Active, not recruiting | Multiple Myeloma | Janssen Research & Development, LLC | May 12, 2014 |
| NCT02195479 | Active, not recruiting | Multiple Myeloma | Janssen Research & Development, LLC | July 21, 2014 |
| NCT02252172 | Active, not recruiting | Multiple Myeloma | Janssen Research & Development, LLC | September 30, 2014 |
| NCT02316106 | Active, not recruiting | Multiple Myeloma | Janssen Research & Development, LLC | December 12, 2014 |
| NCT02336815 | Recruiting | Multiple Myeloma | Karyopharm Therapeutics Inc | January 13, 2015 |
| NCT02343042 | Recruiting | Multiple Myeloma | Karyopharm Therapeutics Inc | January 21, 2015 |
| NCT02431208 | Recruiting | Multiple Myeloma | Hoffmann-La Roche | April 30, 2015 |
| NCT02477891 | Available | Multiple Myeloma | Janssen Research & Development, LLC | June 23, 2015 |
| NCT02497378 | Active, not recruiting | Multiple Myeloma | Janssen Pharmaceutical K.K. | July 14, 2015 |
| NCT02519452 | Active, not recruiting | Multiple Myeloma | Janssen Research & Development, LLC | August 11, 2015 |
| NCT02541383 | Recruiting | Multiple Myeloma | Intergroupe Francophone du Myelome | September 4, 2015 |
| NCT02626481 | Active, not recruiting | Multiple Myeloma | University Hospital, Lille | December 10, 2015 |
| NCT02751255 | Recruiting | Multiple Myeloma | VU University Medical Center | April 26, 2016 |
| NCT02807454 | Active, not recruiting | Multiple Myeloma | Celgene | June 21, 2016 |
| NCT02816476 | Recruiting | Amyloidosis | University Hospital, Limoges | June 28, 2016 |
| NCT02841033 | Recruiting | AL Amyloidosis | Vaishali Sanchorawala | July 21, 2016 |
| NCT02852837 | Recruiting | Multiple Myeloma | Janssen Research & Development, LLC | August 2, 2016 |
| NCT02874742 | Recruiting | Multiple Myeloma | Janssen Research & Development, LLC | August 22, 2016 |
| NCT02918331 | Recruiting | Multiple Myeloma | Janssen Pharmaceutical K.K. | September 28, 2016 |
| NCT02927925 | Recruiting | Lymphoma | Janssen Research & Development, LLC | October 7, 2016 |
| NCT02944565 | Active, not recruiting | Plasma Cell Myeloma | Ohio State University Comprehensive Cancer Center | October 26, 2016 |
| NCT02951819 | Active, not recruiting | Multiple Myeloma | Janssen Scientific Affairs, LLC | November 1, 2016 |
| NCT02955810 | Active, not recruiting | Multiple Myeloma | National University of Ireland, Galway, Ireland | November 4, 2016 |
| NCT02963493 | Recruiting | Multiple Myeloma | Oncopeptides AB | November 15, 2016 |
| NCT02977494 | Recruiting | Multiple Myeloma | University Hospital Tuebingen | November 30, 2016 |
| NCT03011034 | Active, not recruiting | Myelodysplastic Syndromes | Janssen Research & Development, LLC | January 5, 2017 |
| Recruiting | Carcinoma, Non-Small-Cell Lung | Janssen Research & Development, LLC | January 18, 2017 | Recruiting |
| NCT03067571 | Recruiting | Hematopoietic/Lymphoid Cancer, Acute Myelogenous Leukemia, High-Risk Myelodysplastic Syndrome | M.D. Anderson Cancer Center | March 1, 2017 |
| NCT03095118 | Recruiting | Membranoproliferative Glomerulonephritis | Fernando Fervenza | March 29, 2017 |
| NCT03098550 | Recruiting | Advanced Cancer | Bristol-Myers Squibb | March 31, 2017 |
| NCT03143036 | Not yet recruiting | Relapse and / or Refractory Myeloma | National University Hospital, Singapore | May 8, 2017 |
| NCT03158688 | Recruiting | Relapsed or Refractory Multiple Myeloma | Amgen | May 18, 2017 |
| NCT03177460 | Recruiting | Malignant Neoplasms of Male Genital Organs, Prostate Cancer, Adenocarcinoma of the Prostate | M.D. Anderson Cancer Center | June 6, 2017 |
| NCT03180736 | Recruiting | Multiple Myeloma | European Myeloma Network | June 8, 2017 |
| NCT03184194 | Not yet recruiting | Myeloma | VU University Medical Center | June 12, 2017 |
| NCT03187262 | Recruiting | Waldenström Macroglobulinemia | Dana-Farber Cancer Institute | June 14, 2017 |
| NCT03201965 | Recruiting | Amyloidosis | Janssen Research & Development, LLC | June 28, 2017 |
| NCT03207542 | Not yet recruiting | Malignant Neoplasms Stated as Primary Lymphoid Haematopoietic | M.D. Anderson Cancer Center | July 2, 2017 |
| NCT03215524 | Recruiting | Multiple Myeloma | Myeloma Canada Research Network | July 12, 2017 |
| NCT03217812 | Recruiting | Multiple Myeloma | Janssen Research & Development, LLC | July 14, 2017 |
| NCT03221634 | Not yet recruiting | Multiple Myeloma | Merck Sharp & Dohme Corp. | July 18, 2017 |
| NCT03224507 | Recruiting | Multiple Myeloma | University of Alabama at Birmingham | July 21, 2017 |
| NCT03234972 | Recruiting | Multiple Myeloma | Janssen Research & Development, LLC | August 1, 2017 |
| NCT03236428 | Recruiting | Monoclonal Gammopathy, Smoldering Multiple Myeloma | Dana-Farber Cancer Institute | August 1, 2017 |
| NCT03242889 | Active, not recruiting | Multiple Myeloma | Janssen Pharmaceutical K.K. | August 8, 2017 |
| NCT03277105 | Recruiting | Multiple Myeloma | Janssen Research & Development, LLC | September 8, 2017 |
| NCT03283917 | Recruiting | Hematopoietic/Lymphoid Cancer, Amyloid Light Chain (AL) Amyloidosis | M.D. Anderson Cancer Center | September 14, 2017 |
| NCT03290950 | Recruiting | Multiple Myeloma | Memorial Sloan Kettering Cancer Center | September 25, 2017 |
| NCT03301220 | Recruiting | Smoldering Multiple Myeloma | Janssen Research & Development, LLC | October 4, 2017 |
| NCT03311828 | Recruiting | Recurrent Plasma Cell Myeloma | City of Hope Medical Center | October 17, 2017 |
| NCT03314181 | Recruiting | Cancer - Multiple Myeloma | AbbVie | October 19, 2017 |
| NCT03320707 | Recruiting | Healthy | Janssen Research & Development, LLC | October 25, 2017 |
| NCT03346135 | Not yet recruiting | Plasma Cell Myeloma,Secondary Amyloidosis | City of Hope Medical Center | November 17, 2017 |
| NCT03357952 | Recruiting | Multiple Myeloma | Janssen Research & Development, LLC | November 30, 2017 |
| NCT03384654 | Not yet recruiting | Precursor Cell Lymphoblastic Leukemia-Lymphoma | Janssen Research & Development, LLC | December 27, 2017 |
| NCT03412565 | Not yet recruiting | Multiple Myeloma | Janssen Research & Development, LLC | January 26, 2018 |
| NCT03439293 | Not yet recruiting | Multiple Myeloma | Takeda | February 20, 2018 |
| NCT03447808 | Not yet recruiting | Chronic Lymphocytic Leukemia | Jennifer Woyach | February 27, 2018 |
| NCT03450057 | Recruiting | Multiple Myeloma | Hellenic Society of Hematology | March 1, 2018 |
| NCT03473730 | Not yet recruiting | Malignant Neoplasms of Urinary Tract | M.D. Anderson Cancer Center | March 22, 2018 |
| NCT03475628 | Recruiting | Multiple Myeloma | Hellenic Society of Hematology | March 23, 2018 |
| NCT03477539 | Recruiting | Minimal Residual Disease, Plasma Cell Myeloma | Mayo Clinic | March 26, 2018 |
| NCT03481556 | Not yet recruiting | Multiple Myeloma | Oncopeptides AB | March 29, 2018 |
| NCT03490344 | Not yet recruiting | Multiple Myeloma | Memorial Sloan Kettering Cancer Center | April 6, 2018 |
Approved Drugs of Daratumumab**
| INN (trade name) | Therapeutic area | Dosage | Strength | Route | Company | Marketing start | Market |
| Darzalex | Multiple Myeloma | Injection, solution, concentrate | 20 mg/ml | Intravenous | Janssen Biotech, Inc. | 2015-11-16 |

|
| Darzalex | Multiple Myeloma | Injection, solution, concentrate | 20 mg/ml | Intravenous | Janssen Cilag International Nv | 2016-05-20 |

|
| Darzalex | Multiple Myeloma | Injection, solution, concentrate | 20 mg/ml | Intravenous | Janssen Pharmaceuticals | 2016-07-12 |

|
| Darzalex | Multiple Myeloma | Injection, solution, concentrate | 20 mg/ml | Intravenous | Janssen-Cilag Pty Ltd | 2017-07-17 |

|
INN, International nonproprietary name.
Resources
* The table was excerpted from the following website
https://clinicaltrials.gov/ct2/results?cond=&term=daratumumab
** Information presented in the table was collected from the following websites:
https://www.drugbank.ca/drugs/DB09331
http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/004077/human_med_001979.jsp
http://search.tga.gov.au/s/search.html?collection=tga-artg&profile=record&meta_i=281843
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

