Epitumomab Overview
Introduction of Epitumomab
Epitumomab is a novel, promising therapeutic antibody that targets MS4A1, a key protein expressed on the surface of B-cells. By selectively targeting MS4A1, it offers a mechanism of action that allows for more precise treatment with fewer side effects compared to traditional therapies. With increasing interest in precision medicine and immunotherapy, Epitumomab is emerging as a strong candidate for addressing various B-cell malignancies, including those affecting the immune system, such as lymphoma and leukemia.
Introduction of MS4A1 and Its Role in Immunology
MS4A1, commonly called CD20, is a transmembrane glycoprotein primarily expressed on the surface of B-cells, a vital component of the immune system. It plays a pivotal role in B-cell activation, differentiation, and survival, making it a key target for therapeutic interventions, especially in B-cell malignancies such as non-Hodgkin lymphoma (NHL) and chronic lymphocytic leukemia (CLL).
The Structure of MS4A1
MS4A1 is part of a family of proteins known as the MS4A family, which includes several structurally related proteins involved in immune cell signaling. The CD20 protein is a transmembrane protein characterized by four hydrophobic domains that span the lipid bilayer of the cell membrane. As a result, it has both its carboxyl (COOH) and amino (NH2) termini located intracellularly, while it possesses two extracellular loops. These loops are crucial for B-cell function, as they facilitate the activation of various intracellular signaling cascades upon receptor engagement.
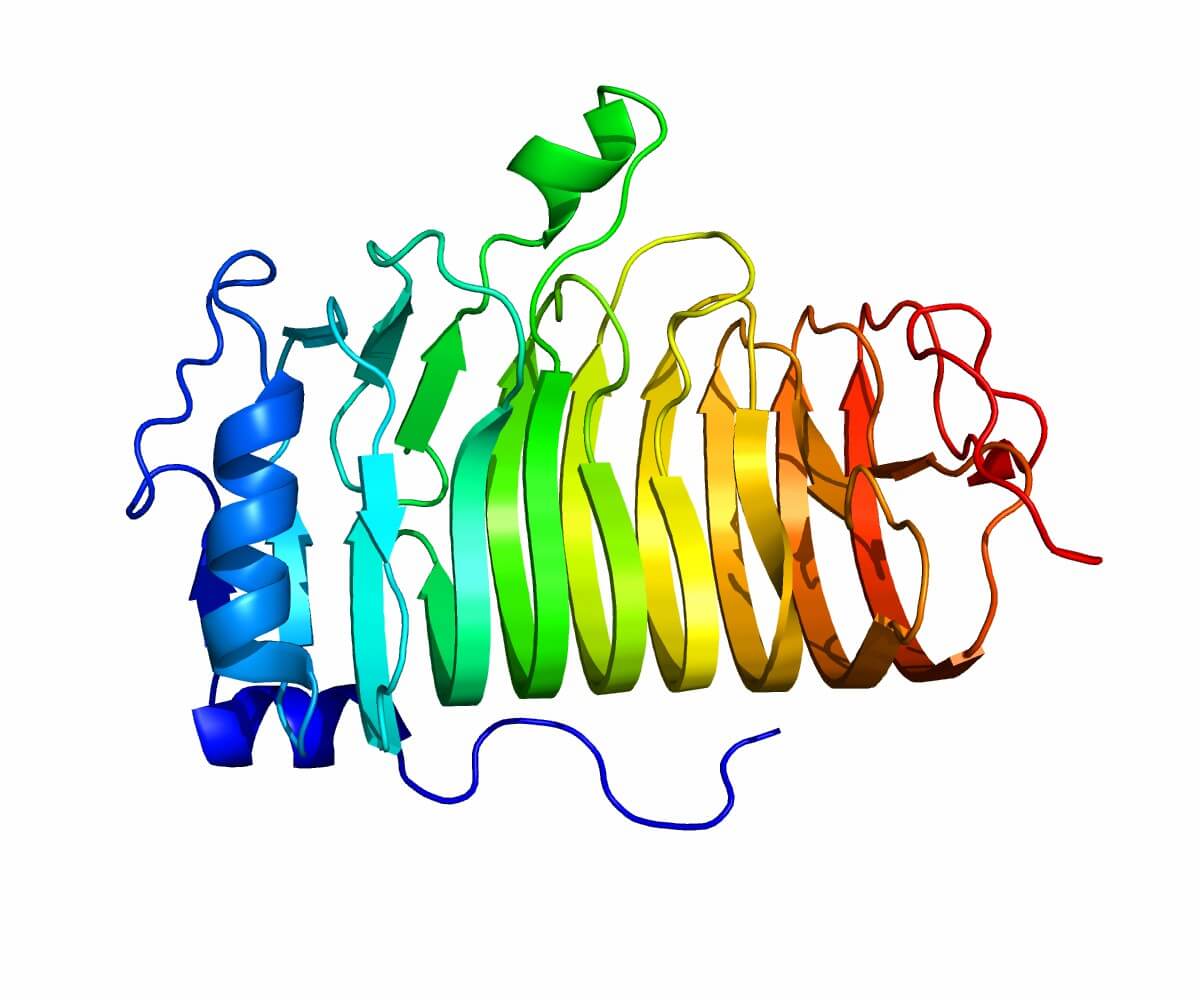 Figure 1. The Structure of CD20. (Wikipedia, Public Domain)
Figure 1. The Structure of CD20. (Wikipedia, Public Domain)
MS4A1 in B-Cell Development and Activation
MS4A1 is expressed during the later stages of B-cell development, from the transitional B-cell stage to mature B-cells. It plays a crucial role in regulating the function of mature B-cells, particularly in their ability to proliferate, differentiate, and survive. Upon activation by external signals such as antigen binding, MS4A1 assists in initiating intracellular signaling pathways that are essential for B-cell activation and the subsequent immune response. The precise mechanisms by which MS4A1 regulates B-cell activation remain an area of active research, but it is clear that its role extends beyond simple membrane anchoring. MS4A1 is believed to participate in the modulation of B-cell receptor (BCR) signaling and contribute to the overall responsiveness of B-cells to external stimuli, including antigens and cytokines.
MS4A1 in B-Cell Malignancies
Given its widespread expression on mature B-cells, MS4A1 becomes a valuable target in the context of B-cell malignancies. Many B-cell cancers, such as non-Hodgkin lymphoma (NHL), CLL, and acute lymphoblastic leukemia (ALL), involve the uncontrolled proliferation of B-cells, often with overexpression or dysregulation of MS4A1. Targeting MS4A1 can lead to the depletion of these malignant B-cells, offering a therapeutic approach that is both specific and effective. MS4A1 is expressed on normal B-cells as well as malignant B-cells, therapeutic interventions targeting CD20 (like Epitumomab) must be carefully considered to balance efficacy with the potential for immune suppression. Nonetheless, the therapeutic targeting of MS4A1 has proven to be highly effective in many cases, leading to reduced tumor burden and extended survival in patients with B-cell malignancies.
The Mechanism of Epitumomab Action
Epitumomab works by binding with high affinity to MS4A1, initiating a cascade of immune responses aimed at the destruction of B-cells. The first step involves the monoclonal antibody, Epitumomab, binding to the extracellular domain of MS4A1. This interaction is highly specific, meaning that Epitumomab primarily targets cells expressing this protein, which are mainly B-cells. The binding is not only highly selective but also facilitates the subsequent immune responses by providing a clear signal for immune cells to recognize and attack. Once Epitumomab binds to MS4A1, it can mediate cell death through several mechanisms, primarily by inducing apoptosis or programmed cell death. This process is initiated by the signaling pathways triggered through antibody binding. The engagement of MS4A1 can lead to the activation of caspases, which are enzymes responsible for executing apoptosis. This is particularly effective in B-cell malignancies, where the malignant B-cells are often resistant to other forms of cell death.
Epitumomab's ability to induce cell death is enhanced by the immune system's natural mechanisms. One of the key processes involved is antibody-dependent cellular cytotoxicity (ADCC). In ADCC, immune effector cells such as natural killer (NK) cells or macrophages recognize the antibody bound to the target B-cell and release cytotoxic molecules that kill the malignant cell. This enhances the therapeutic potential of Epitumomab by amplifying its effects through the body's immune system. Another mechanism by which Epitumomab exerts its action is through complement-dependent cytotoxicity (CDC). After Epitumomab binds to MS4A1, it can activate the complement system, a part of the immune system that plays a critical role in defending against pathogens and cancer cells. The complement system triggers a series of events that culminate in the formation of a membrane attack complex (MAC) that disrupts the target cell's membrane, leading to cell lysis.
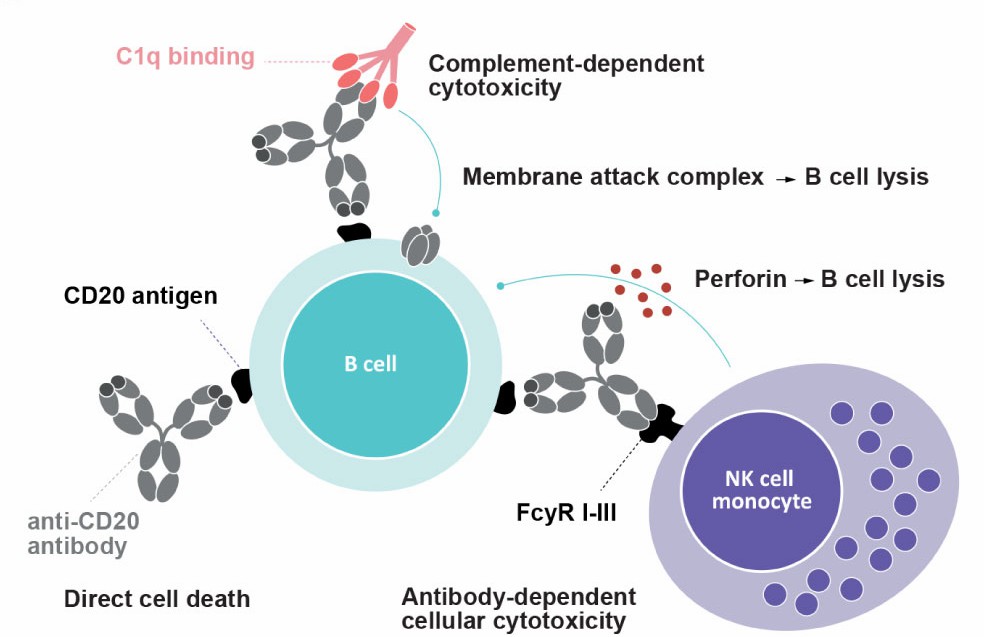 Figure 2. Schematic Representation of Different Mechanisms of Action Involved upon Anti-CD20 mAb Binding1,2.
Figure 2. Schematic Representation of Different Mechanisms of Action Involved upon Anti-CD20 mAb Binding1,2.
The Clinical Applications of Epitumomab
Epitumomab's targeting of MS4A1 makes it a valuable tool in the treatment of various B-cell malignancies, including non-Hodgkin lymphoma, chronic lymphocytic leukemia (CLL), and acute lymphoblastic leukemia (ALL). These cancers are typically characterized by the proliferation of abnormal B-cells, and the ability of Epitumomab to selectively target and kill these cells offers an alternative to more generalized treatments like chemotherapy.
Non-Hodgkin lymphoma (NHL) represents a diverse group of B-cell-related cancers that are difficult to treat effectively using traditional therapies. By targeting the MS4A1 protein, Epitumomab provides a precise method for reducing the number of malignant B-cells in the body. Clinical trials have shown promising results, with patients exhibiting improved response rates and fewer side effects compared to conventional treatments.
Chronic lymphocytic leukemia is another B-cell malignancy that benefits from MS4A1-targeted therapies. Epitumomab's ability to target the CD20 protein on the surface of leukemic B-cells helps to reduce tumor burden and slow the progression of the disease. Given its selective targeting mechanism, Epitumomab provides a potential therapeutic option for patients who may not respond well to other forms of treatment.
What We Provide
Anti-Human MS4A1 Recombinant Antibody (Epitumomab)
We provide high-quality Epitumomab for use in IP, IF, FuncS, FC, Neut, ELISA, ICC, and most other immunological methods. The product is for lab research use only, not for diagnostic, therapeutic, or any in vivo human use.
- Host Species
- Mouse
- Derivation
- Mouse
- Type
- IgG2a
- Specificity
- MS4A1(membrane-spanning 4-domains subfamily A member 1, CD20) [Homo sapiens]
- Species Reactivity
- Human
- Applications
- Suitable for use in IP, IF, FuncS, FC, Neut, ELISA, ICC and most other immunological methods.
- CAS
- 263547-71-3
- Generic Name
- epitumomab
- Related Disease
- Unknown
- de Sèze, Jérôme, et al. "Anti-CD20 therapies in multiple sclerosis: From pathology to the clinic." Frontiers in immunology 14 (2023): 1004795.
- The image retrieved from Figure 2 "Anti-CD20 therapies in multiple sclerosis: From pathology to the clinic." de Sèze, 2023, is used under [CC BY 4.0]. The image is cropped to remain part C.
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.



