Erenumab Overview
Introduction of Erenumab
Erenumab (AMG-334) is a human IgG2λ monoclonal antibody designed specifically to bind and antagonize the calcitonin gene-related peptide receptor (CGRPR) as a means to prevent migraines. Erenumab is in fact a novel therapeutic approach as the first and only U.S. Food and Drug Administration (FDA) approved for prevention of migraine in adults.
Mechanism of Action of Erenumab
During a migraine, activated primary sensory neurons (meningeal nociceptors) in the trigeminal ganglion release calcitonin gene-related peptide (CGRP) from their peripherally projecting nerve endings located within the meninges. This CGRP then binds to and activates CGRPR located around meningeal vessels, causing vasodilation, mast cell degranulation, and plasma extravasation. CGRPR is a complex of calcitonin receptor- like receptor (CALCRL) and receptor activity- modifying protein 1 (RAMP1) which includes the CGRP binding pocket. Erenumab targets the extracellular domains of CGRPR, blocking the CGRP-CGRPR signaling, and thereby behaves its efficacy in treatment of migraine.
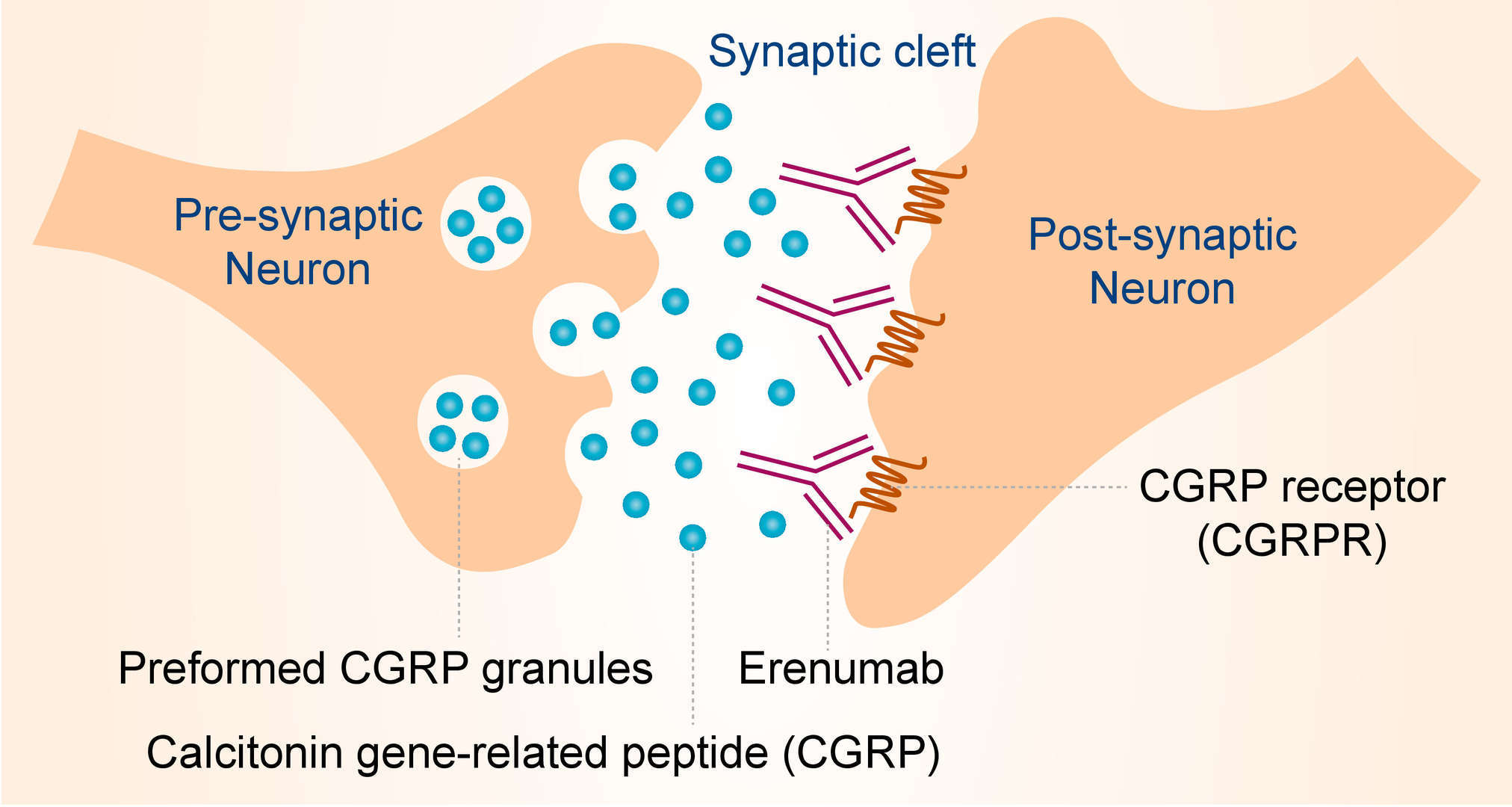 Fig.1 Mechanism of Action of Erenumab
Fig.1 Mechanism of Action of Erenumab
Clinical Projects of Erenumab*
| NCT ID | Status | Conditions | Lead Sponsor | Update Time |
| NCT03333109 | Recruiting | Migraine | Novartis Pharmaceuticals | November 6, 2017 |
| NCT03096834 | Recruiting | Episodic Migraine | Novartis Pharmaceuticals | March 30, 2017 |
Approved Drugs of Erenumab**
| INN (trade name) | Therapeutic area | Dose | Strength | Route | Company | Marketing start | Market |
| Aimovig | Migraine | Injection, Solution | 10 mg/mL | Intravenous | Amgen Inc. | May 17, 2018 |

|
Resources
* The table was excerpted from the following website
https://clinicaltrials.gov/ct2/results?cond=&term=Erenumab
** Information presented in the table were collected from the following website:
https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=761077
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

