Firivumab Overview
Introduction of Firivumab
Firivumab (CT-P22, CT120) is a novel monoclonal antibody (mAb) with great promise for the treatment of viral infections, especially influenza viruses. It is specifically designed to target HA in influenza A virus (IAV) and has been shown to neutralize IVA subtypes H1N1, H5N1, H6N1, H6N2, H8N4, H8N8, H9N2, and H12N7. Pre-clinical studies have confirmed its remarkable efficacy in defending mice against H1N1. The fact that it can prevent viral entry via membrane fusion makes it a hopeful therapeutic tool in the fight against viral disease.
Biological and Chemical Properties of Firivumab
Hemagglutinin (influenza) Structure
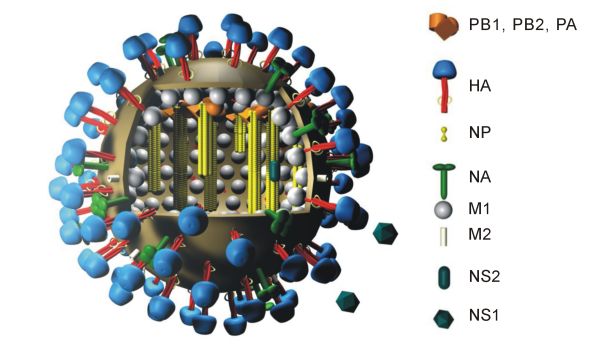 Figure 1. The Structure of Influenza (Wikipedia, Public Domain)
Figure 1. The Structure of Influenza (Wikipedia, Public Domain)
Protein Chemical Formula
C6472H9982N1732O2014S44
Protein Average Weight
The average weight of the protein is approximately 146000.0 Da.
The Mechanism of Firivumab Action
Firivumab binds to epitopes on viral proteins including hemagglutinin (HA), important for the life of the virus. This action targets a class I fusion protein, hemagglutinin, which binds the virus to host cells and then fuses the viral membrane with the host cell membrane. This fusion is one of the main mechanisms that allow the virus to bring its genes into the host cell and initiate replication.
The Role of Hemagglutinin in Viral Entry
Hemagglutinin, a glycoprotein found on influenza viruses' surfaces, is a homotrimeric glycoprotein essential for virus viability. HA monomers consist of two subdomains, HA1 and HA2. HA1 facilitates the virus's attachment to sialic acid receptors on host cell surfaces, an important stage in viral entry. The HA2 domain, which bridges viral and host membranes, is activated in response to low pH in the endosome. Firivumab disrupts these structures by targeting the highly conserved stem or stalk part of the HA protein. This method prevents the conformational modifications needed for the fusion of viral and host membranes, which render the virus incapable of infecting host cells. By binding to the HA stem region, firivumab is able to neutralize various virus subtypes, including H1N1 and H5N1, commonly associated with seasonal flu and bird flu.
Specificity and Broad Neutralization: A Key Feature
What sets firivumab apart from other antiviral agents is its broad-spectrum action against multiple strains of influenza. This efficacy is largely due to its targeting of the HA2 domain, which is more conserved than the receptor-binding HA1 domain. The diversity in HA subtypes (H1 through H18) poses significant challenges in vaccine and drug development. However, firivumab's ability to recognize the conserved stem region provides it with the potential to neutralize various influenza A virus strains. For example, monoclonal antibodies such as FI6 and CR6261, which also target the HA stem, have shown cross-neutralization activity against both human and avian influenza virus strains. Firivumab employs similar principles, inhibiting membrane fusion and preventing viral entry into host cells, thereby offering a promising avenue for antiviral therapy.
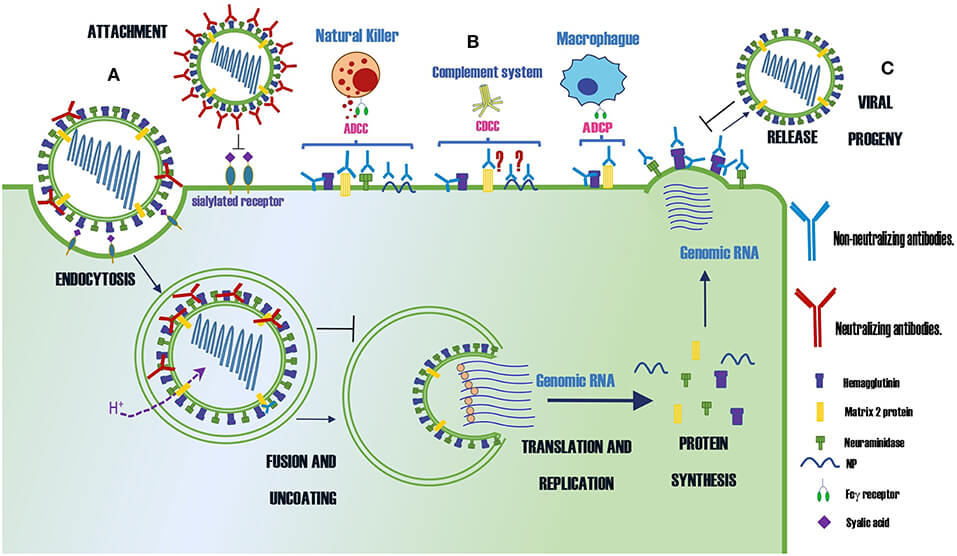 Figure 2. Summary of the Protection Mechanisms of Neutralizing and Non-neutralizing Abs Specific for Different Proteins of the Influenza Virus1.
Figure 2. Summary of the Protection Mechanisms of Neutralizing and Non-neutralizing Abs Specific for Different Proteins of the Influenza Virus1.
The Clinical Applications of Firivumab
Firivumab's potential therapeutic applications are vast, especially in the context of viral infections. The monoclonal antibody has shown promise in preclinical models of influenza, demonstrating its ability to reduce viral load and improve clinical outcomes. Its mechanism of action, focusing on blocking the fusion process, provides a therapeutic strategy applicable across various influenza subtypes, including those resistant to existing antiviral drugs.
Broad Application Against Influenza Viruses
Given the global health burden posed by influenza, firivumab could play a crucial role in combating seasonal outbreaks and potentially even pandemic strains. Influenza viruses are notorious for their ability to mutate, particularly in the hemagglutinin and neuraminidase proteins. These mutations can lead to antigenic drift, making it difficult for the immune system and vaccines to mount an effective response. By targeting the more conserved HA stem region, firivumab overcomes this challenge, offering protection against multiple viral strains, including those resistant to other antiviral treatments.
In addition to its application in influenza, firivumab could be adapted for use against other viral infections, such as avian influenza (H5N1) or swine-origin influenza (H1N1), which are less common but potentially more devastating. The monoclonal antibody's broad-spectrum efficacy against various subtypes is a major advantage in pandemic preparedness.
Synergy with Other Antiviral Agents
Firivumab could also be used in combination with other antiviral agents to enhance therapeutic efficacy. For instance, pairing firivumab with neuraminidase inhibitors like oseltamivir or zanamivir could provide a more comprehensive approach to influenza treatment. While neuraminidase inhibitors prevent the release of new viral particles from infected cells, firivumab blocks viral entry, preventing initial infection. This combination therapy could provide more effective treatment and reduce the likelihood of viral resistance.
What We Provide
Anti-IAV HA Recombinant Antibody (Firivumab)
We provide high-quality firivumab for use in ELISA, FC, IP, FuncS, IF, Neut, ICC and most other immunological methods. This product is for lab research use only, not for diagnostic, therapeutic, or any in vivo human use.
- Host Species
- human
- Derivation
- human
- Type
- IgG1 - kappa
- Specificity
- influenza A virus hemagglutinin HA [unidentified influenza virus]
- Species Reactivity
- IAV
- Applications
- Suitable for use in ELISA, FC, IP, FuncS, IF, Neut, ICC and most other immunological methods.
- CAS
- 1443004-15-6
- Generic Name
- firivumab
- Related Disease
- Influenza A infection
- Padilla-Quirarte, Herbey O., et al. "Protective antibodies against influenza proteins." Frontiers in immunology 10 (2019): 1677. Distributed under Open Access license CC BY 4.0, without modification.
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.



