Galiximab Overview
Introduction of Galiximab
Galiximab is a primatized IgG1λ monoclonal antibody (mAb) for the treatment of B-cell lymphoma (BCL). It is a chimeric antibody consisting of human constant and primate (cynomolgus macaque) variable regions. This ‘primatized’ antibody is structurally indistinguishable from human antibodies and, therefore, is not significantly immunogenic in humans. It binds specifically to CD80, which is transiently expressed on the surface of activated B cells and antigen-presenting cells (APCs), including dendritic cells, but is constitutively expressed on a variety of Hodgkin’s lymphomas (NHLs).
Mechanism of Action of Galiximab
CD80, also known as B7.1, is a surface glycoprotein and a member of the expanding B7-family of co-stimulatory molecules. CD80, and the structurally and functionally related CD86, belong to the B7-family of co-stimulatory molecules providing critical co-stimulatory and co-inhibitory signals that regulate T-cell-mediated immunity. CD80 and CD86 antigens have a restricted expression pattern, being expressed mainly on APC, a subset of normal B cells, as well as on various subtypes of B-cell lymphomas, but rarely on stromal cells and non-hematological cancer cells. Of note, both of these antigens play a major role in providing co-stimulatory signals to T cells, leading to their proliferation, cytokine production and development of effector functions. CD80 and CD86 regulate T-cell function through their interaction with CD28 (low affinity) and CD152 (high affinity) antigens present on unstimulated (causing activation) and activated (causing inhibition) T cells, respectively. Under normal circumstances, the interaction of a CD28+ T cell with CD80 or CD86 results in activation of the T cell with cell-cycle progression, survival and cytokine production. T-cell activation results in the expression of a second member of the CD28 family: CD152, also known as cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4). CTLA-4 has a higher affinity for CD80/CD86 and, on binding to CD80 or CD86, mediates various inhibitory signaling events that counteract CD28- mediated co-stimulation.
Preclinical in vitro and in vivo studies with galiximab have been encouraging, showing significant antitumor activity against various B-cell lymphoma cell lines, both as a single agent as well as in combination with rituximab. The mechanisms of action of galiximab are largely unknown. However, these preclinical studies with galiximab have demonstrated an inhibition of cell proliferation, increase in apoptosis with upregulation of pro-apoptotic molecules and induction of antibody-dependent cell-mediated cytotoxicity (ADCC) against various B-cell lymphoma cell lines. The ADCC activity was higher when galiximab was combined with rituximab. In severe combined immunodeficiency mice with human SKW lymphoma xenografts, which express both CD80 and CD20, galiximab and rituximab were similar in their capability to prevent tumor progression and improve survival. The combination of galiximab and rituximab extended survival beyond that observed with either antibody alone. In addition, other investigators have demonstrated that galiximab (on binding CD80) affects CD80-CD28 and, to a lesser degree, CD80-CD152 interactions. It has been postulated that by altering CD80-CD152 interactions, galiximab might elucidate T-cell responses against B-cell lymphomas, thus, creating an antitumor microenvironment.
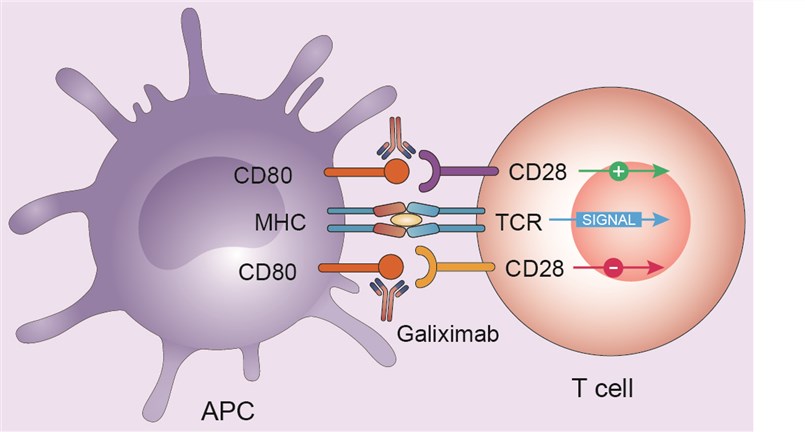 Fig.1 Mechanism of action of galiximab
Fig.1 Mechanism of action of galiximab
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.



