Nemolizumab Overview
Introduction of Nemolizumab
Nemolizumab (CIM331) is an experimental drug for the treatment of itching in people with atopic dermatitis (AD). It is a humanized monoclonal antibody (mAb) that blocks the interleukin-31 receptor A (IL-13RA). In a phase 1 clinical study, a single dose of nemolizumab, administered subcutaneously, suppressed pruritus, consequently improved sleep disturbance, and reduced concomitant use of topical glucocorticoids in adult patients with moderate-to-severe atopic dermatitis. In this phase 2 trial, nemolizumab at all monthly doses significantly improved pruritus in patients with moderate-to-severe atopic dermatitis, which showed the efficacy of targeting IL-31RA.
Mechanism of Action of Nemolizumab
The pathology of AD is closely related to IL-31, a cytokine with an anti-parallel four-helix bundle structure in the gp130/IL-6 cytokine family. IL-31 is mainly produced from T helper 2 (TH2) cells. In steady-state TH2 cells, the endothelial PAS domain-containing protein 1 (EPAS1) molecule is associated with macrophage-stimulating 1 (MST1) and dedicator of cytokinesis 8 (DOCK8). When activated (eg, by staphylococcal enterotoxin B), EPAS1 translocates from the cytoplasm into nucleus and heterodimerizes with another transcription factor SP1. The EPAS1/SP1 heterodimer binds to the promoter region of IL-31 and initiates transcription. IL-31 signals via a receptor complex that is composed of IL-31 receptor A (IL31RA) and oncostatin M receptor (OSMR) subunits. These receptor subunits are expressed in activated monocytes and in unstimulated epithelial cells. IL-31RA binds IL-31 through its cytokine binding domain (CBD). OSMR does not normally bind to IL-31 but it does increase the IL-31 binding affinity to IL-31RA. IL-31RA has a intracellular domain that possesses a box1 motif that mediates association with kinases of the JAK family. Additionally, the intracellular portion of the IL-31RA contains tyrosine residues. When IL-31 binds to the receptor complex, JAK kinases are activated which phosphorylate and activate STAT1, STAT3, and STAT5. The OSMR portion of the IL-31 binding complex contains intracellular motifs box1 and box2. This allows for Janus kinase (JAK) 1 and JAK2 to bind, which are recruited once the tyrosine residues on the intracellular domain are phosphorylated. Through these phosphorylation sites, Signal transducer and activator of transcription (STAT) 3 and STAT5 are recruited and phosphorylated by JAK1 and JAK2. In addition to STATs, phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K) is recruited, which stimulates the PI3K/protein kinase B (PKB, also known as AKT) signaling pathway. In contrast to IL-31RA, which binds Src homology 2 domain-containing phosphatase-2 (SHP-2), the OSMR interacts with the adaptor protein Shc via the phosphorylated tyrosines on its intracellular domain. Through Shc, the RAS/RAF/mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinases (ERK) pathway is activated along with the p38 and JNK pathways. When IL-31 binds to the IL-31RA/OSMR complex, the JAK, PI3K/AKT, and ERK signaling pathways are activated. In addition, the IL-31RA+ sensory nerves coexpress transient receptor potential cation channel vanilloid subtype 1 (TRPV1) and transient receptor potential cation channel ankyrin subtype 1(TRPA1). IL-31 also facilitates the elongation and branching of sensory nerves and increases the expression of IL-1a, CCL17, CCL22, S100A7, and b-defensing 2 and decreases the expression of filaggrin and corneodesmosin. AD shares all of these pathologic responses. Nemolizumab targets the IL-31RA and blocks the IL-31-envolved downstream pathways, thereby shows its’ therapeutic efficacy for AD.
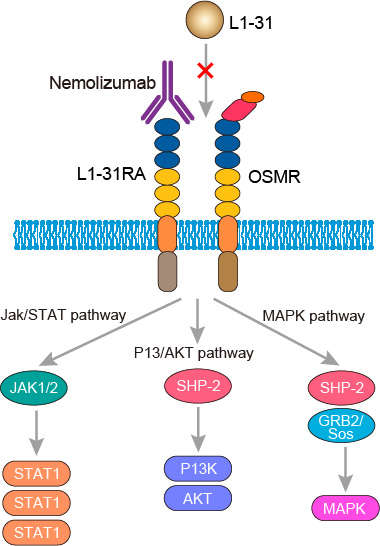 Fig.1 Mechanism of action of nemolizumab
Fig.1 Mechanism of action of nemolizumab
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

