Ocrelizumab Overview
Introduction of Ocrelizumab
Ocrelizumab is a novel humanized antibody targeting the B lymphocytes that express the CD20 antigen. It is designed through recombinant DNA technology and has shown promising results in the treatment of relapsing (RMS) or primary progressive (PPMS) forms of multiple sclerosis, rheumatoid arthritis, lupus erythematosus and hematological cancer. Due to its mostly human origin, ocrelizumab is expected to induce less immunogenicity and theoretically is expected to cause fewer anti-drug antibodies to be formed, as well as milder infusion reactions. OCREVUS™, the first ocrelizumab drug developed by Genentech/Roche for intravenous injection has been successively approved by Food and Drug Administration (in March 2017), Health Canada (in August 2017), and European Medicines Agency (in January 2018) for the treatment of multiple sclerosis.
Mechanism of Action of Ocrelizumab
The precise mechanism by which ocrelizumab exerts its therapeutic effects in multiple sclerosis is unknown, but is presumed to involve binding to CD20, a cell surface antigen present on pre-, naïve, mature, and memory B cells, which are known to contribute to the pathogenesis of multiple sclerosis through activation of pro-inflammatory T cells and secretion of proinflammatory cytokines. Following cell surface binding to B lymphocytes, ocrelizumab results in antibody-dependent cellular cytolysis and complement-mediated lysis involving macrophages, natural killer cells, and cytotoxic T cells that act together to cause cell death. Another mechanism is apoptosis, which may result from cross-linking membrane CD20 on the target cell surface.
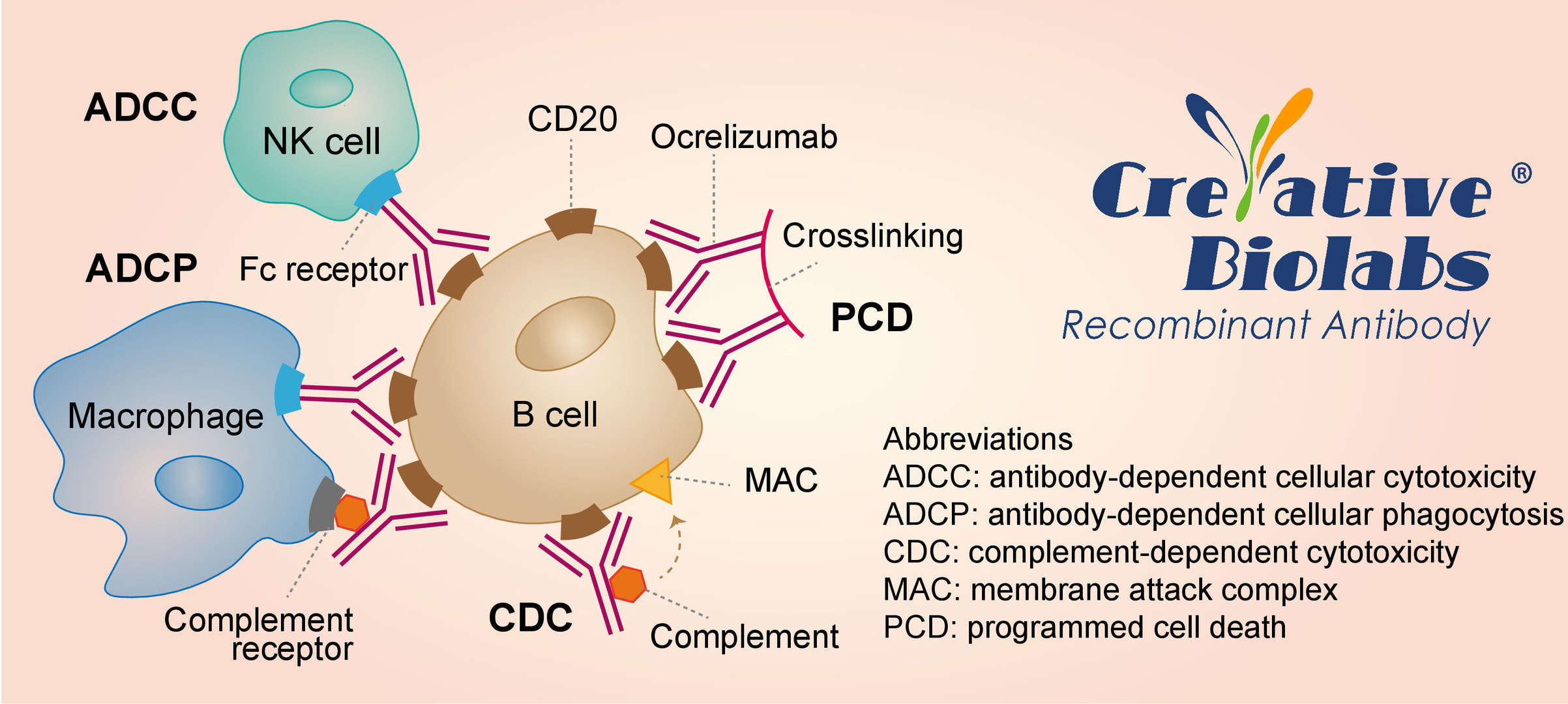 Fig. 1 Mechanism of Action of Ocrelizumab
Fig. 1 Mechanism of Action of Ocrelizumab
The image shows the various mechanism in the pathogenesis of multiple sclerosis and the various drugs that are being developed as potential therapies for multiple sclerosis.
Clinical Projects of Ocrelizumab*
| NCT ID | Status | Conditions | Lead Sponsor | Update Time |
| NCT03085810 | Recruiting | Multiple Sclerosis, Relapsing-Remitting | Hoffmann-La Roche | March 21, 2017 |
| NCT02637856 | Recruiting | Multiple Sclerosis, Relapsing-Remitting | Genentech, Inc. | December 22, 2015 |
| NCT03025269 | Enrolling by invitation | Multiple Sclerosis | University at Buffalo | January 19, 2017 |
| NCT02688985 | Recruiting | Relapsing Multiple Sclerosis | Genentech, Inc. | February 23, 2016 |
| NCT03344094 | Recruiting | Multiple Sclerosis | University of Chicago | November 17, 2017 |
| NCT02980042 | Recruiting | Multiple Sclerosis | University of Colorado, Denver | December 2, 2016 |
| NCT01194570 | Active, not recruiting | Multiple Sclerosis, Primary Progressive | Hoffmann-La Roche | September 3, 2010 |
| NCT03157830 | Recruiting | Relapsing Remitting Multiple Sclerosis | Providence Health & Services | May 17, 2017 |
| NCT01412333 | Active, not recruiting | Relapsing Multiple Sclerosis | Hoffmann-La Roche | August 9, 2011 |
| NCT03396822 | Not yet recruiting | Multiple Sclerosis | University of Maryland | January 11, 2018 |
| NCT00676715 | Active, not recruiting | Multiple Sclerosis, Relapsing-Remitting | Genentech, Inc. | May 13, 2008 |
| NCT03138525 | Recruiting | Multiple Sclerosis | Brigham and Women's Hospital | May 3, 2017 |
| NCT02545868 | Active, not recruiting | Multiple Sclerosis, Relapsing-Remitting | Hoffmann-La Roche | September 10, 2015 |
| NCT00476996 | Active, not recruiting | Rheumatoid Arthritis | Genentech, Inc. | May 22, 2007 |
Approved Drugs of Ocrelizumab**
| INN (trade name) | Therapeutic area | Dosage | Strength | Route | Company | Marketing start | Market |
| Ocrevus | Multiple Sclerosis | Injection | 300 mg/10mL | Intravenous | Genentech, Inc. | March 28, 2017 |

|
| Ocrevus | Multiple Sclerosis | Solution | 30 mg | Intravenous | Hoffmann La Roche | September 21, 2017 |

|
| Ocrevus | Multiple Sclerosis | Injection | 300 mg/10mL | Intravenous | Roche Registration Limited | January 8, 2018 |

|
INN, International nonproprietary name.
What We Provide
Therapeutic Antibody
Ocrelizumab
We provide high-quality ocrelizumab for use in WB, FC, IP, ELISA, Neut, FuncS, IF and most other immunological methods. For lab research use only, not for diagnostic, therapeutic or any in vivo human use.
Resources
* The table was excerpted from the following website
https://clinicaltrials.gov/ct2/results?cond=&term=Ocrelizumab
** Information presented in the table was collected from the following websites:
https://www.drugbank.ca/drugs/DB11988
http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/004043/human_med_002187.jsp
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.


