Oleclumab Overview
Introduction of Oleclumab
Oleclumab (as known as MEDI9447) is a human monoclonal antibody (mAb) being investigated for the treatment of various types of cancer like Solid Tumors, Pancreatic and Colorectal cancer. It is a monoclonal antibody against the ectoenzyme CD73 (cluster of differentiation 73), also known as 5'-nucleotidase (5'-NT; ecto-5'-nucleotidase; NT5E) with potential antineoplastic activity. In contrast with many other cancer immunotherapy agents such as checkpoint inhibitors or T cell agonists, MEDI9447 drives changes in both myeloid and lymphoid infiltrating leukocyte populations within the tumor microenvironment. Changes include significant increases in CD8 effector cells and activated macrophages, as well as a reduction in the proportions of myeloid-derived suppressor cells (MDSC) and regulatory T lymphocytes. Furthermore, these changes correlate directly with responder and non-responder subpopulations within the arms of animal studies using syngeneic tumors. Data showing additive activity between MEDI9447 and other immune-mediated therapy antibodies demonstrates the importance of relieving adenosine-mediated immunosuppression within tumors.
Mechanism of Action of Oleclumab
CD73 is a glycophosphatidylinositol-anchored receptor found on tumor cells as well as on stromal cells such as endothelial cells and certain leukocytes. This enzyme catalyzes the conversion of adenosine monophosphate (AMP) to adenosine and organic phosphate. Binding of adenosine to the extracellular portion of adenosine receptors triggers signaling through cyclic AMP to inhibit T-cell receptor activation. CD73 is believed to play a role in mediating the inhibitory function of regulatory B and T lymphocytes 2 as well as in maintaining endothelial integrity. In addition to their roles in the biology of healthy tissue, CD73 and adenosine each affect tumor biology. The presence of extracellular adenosine within the tumor microenvironment has been described as an immunosuppressive “halo” surrounding the tumor and permeating the tumor microenvironment and preventing antitumor immunity. Consistent with this role for adenosine, knockout mice lacking adenosine receptors have been shown to reject tumors more readily than normal mice and knockout mice lacking CD73 have increased antitumor immunity and show decreased carcinogenesis when compared with normal mice. Specifically, extracellular adenosine is believed to mediate the immunosuppressive effects of both regulatory T cells and myeloid-derived suppressor cells (MDSCs), among others. Recent reports showing tumor growth inhibition with adenosine receptor inhibitors further support a role for adenosine in promoting tumor growth. Taken together with other studies showing that molecular inhibition of CD73 with small molecules or antibodies can inhibit tumor formation, growth, and metastasis, it has been hypothesized that tumors use CD73 to generate adenosine and, thereby, suppress antitumor immunity. MEDI9447 is a human IgG1λ monoclonal antibody that selectively binds to and inhibits the ectonucleotidase activity of CD73. Upon administration, oleclumab targets and binds to CD73, leading to clustering of and internalization of CD73. This prevents CD73-mediated conversion of adenosine monophosphate (AMP) to adenosine and decreases the amount of free adenosine. This prevents adenosine-mediated lymphocyte suppression and increases the activity of CD8-positive effector cells. This also activates macrophages, and reduces both myeloid-derived suppressor cells (MDSCs) and regulatory T-lymphocytes. By abrogating the inhibitory effect on the immune system and enhancing the cytotoxic T-cell-mediated immune response against cancer cells, tumor cell growth decreases. In addition, clustering and internalization of CD73 decreases the migration of cancer cells and prevents metastasis.
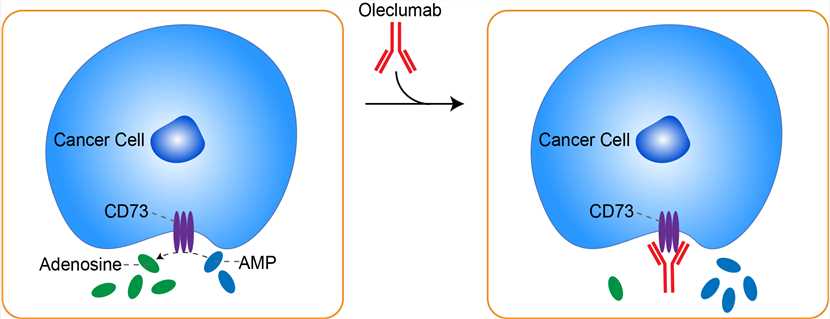
Fig 1. Mechanism of Action of Oleclumab
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

