Radretumab Overview
Introduction of Radretumab
Radretumab, also termed 131I-L19-SIP, is a radioimmunotherapy agent developed by Philogen primarily for the treatment of Hodgkin's lymphoma (HL) and other hematologic malignancies. It is a conjugate composed of two parts: the antibody part, specifically designed to target the extra domain-B (EDB) of fibronectin, and the radioactive component, Iodine-131. Fibronectin, encoded by the FN1 gene located on chromosome 2q34, is a crucial component of the extracellular matrix involved in cell adhesion, growth, migration, and differentiation. The molecule exists in multiple isoforms due to alternative splicing of its pre-mRNA, producing variants like the extra domain-A (ED-A) and extra domain-B (ED-B). Unlike the more ubiquitous forms of fibronectin, ED-B fibronectin is typically absent in normal tissues but is prominently expressed in proliferating vasculature during pathological conditions. This ED-B variant facilitates distinctive interactions in the cellular microenvironment during pathological processes without altering the fundamental binding activities of fibronectin, making it a significant target for both diagnostic and therapeutic applications in oncology and related conditions. The aberrant expression of ED-B fibronectin is notably observed in various diseases, including breast cancer, glioblastoma, colorectal cancer, fibrosis, and some chronic inflammatory diseases. After several trials in solid cancers and brain metastasis indications, radretumab is currently in Phase II of clinical development worldwide, particularly for the treatment of Hodgkin's lymphoma and other hematologic tumors. Radretumab represents a promising biotherapeutic agent that exploits the pathological over-expression of ED-B fibronectin for targeted cancer therapy, offering potential for diagnostic imaging, direct therapeutic effects, and as a delivery system for cytotoxic agents.
Biological Properties of Radretumab
Protein Structure
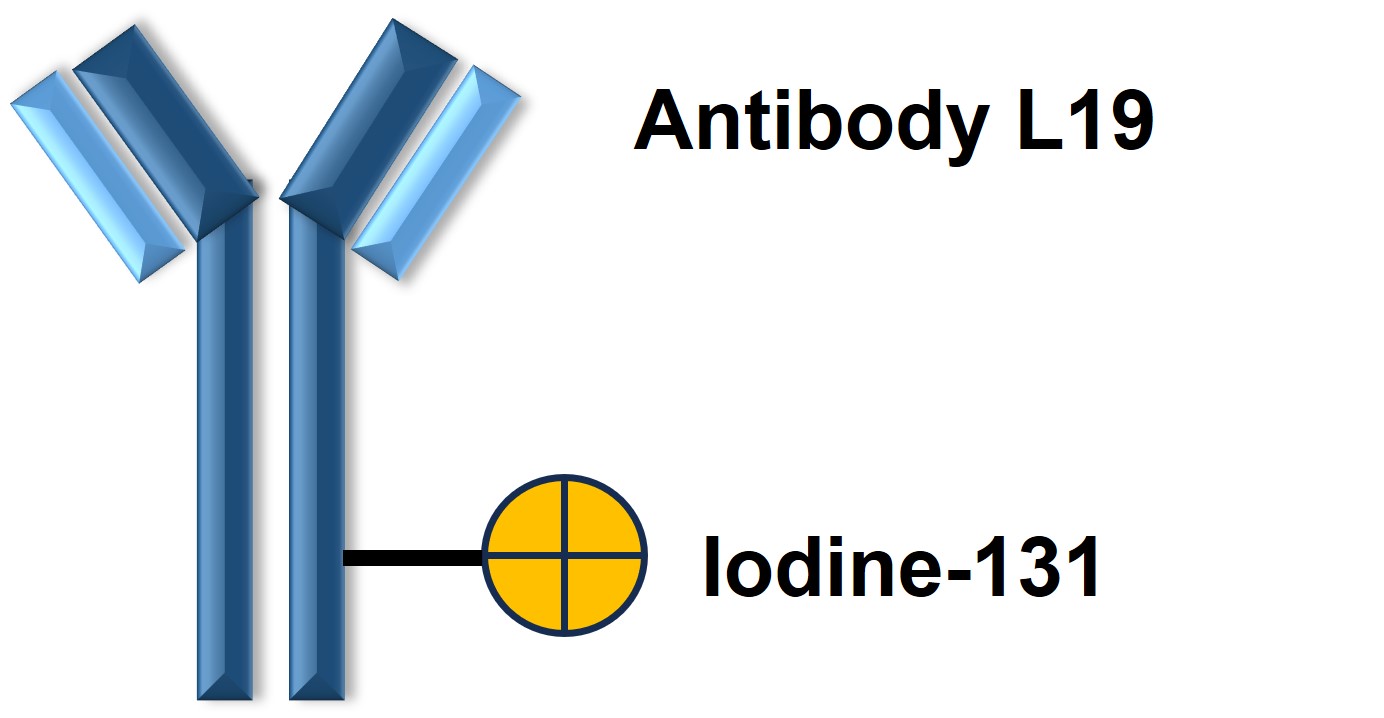 Figure 1. The Structure of Radretumab (Creative Biolabs Original)
Figure 1. The Structure of Radretumab (Creative Biolabs Original)
The Mechanism of Radretumab Action
In normal physiological states, ED-B fibronectin is scarcely detectable after embryonic development. However, its expression is markedly upregulated in pathological conditions involving extensive tissue remodeling, such as cancer, chronic inflammation, and fibrosis. In tumors, ED-B fibronectin is particularly associated with angiogenic vasculature, making it a compelling target for anti-tumor strategies. For example, in the treatment of Hodgkin's lymphoma, radretumab can specifically target and bind to the ED-B variant of fibronectin involved in tumor angiogenesis, thereby inhibiting tumor vascularization and restricting tumor growth and spread. In addition to its role in inhibiting tumor angiogenesis, radretumab also utilizes the radiation emitted by its radioactive isotope to directly kill tumor cells, enhancing the therapeutic effect. In a trial involving patients with relapsed or refractory Hodgkin's lymphoma, selective tumor uptake was observed in 14 of 18 patients treated with radretumab, with 11 meeting the criteria for radioimmunotherapy. Among these 11 patients, 3 achieved complete remission, and 1 achieved partial remission, while the condition of the other treated patients remained stable. The overall objective response rate reached 40%, and any grade 3-4 thrombocytopenia or leukopenia that occurred during treatment was controllable. In summary, radretumab has shown potential efficacy in some patients with relapsed or refractory Hodgkin's lymphoma, providing a novel treatment strategy for tumors that are unresponsive to conventional therapies.
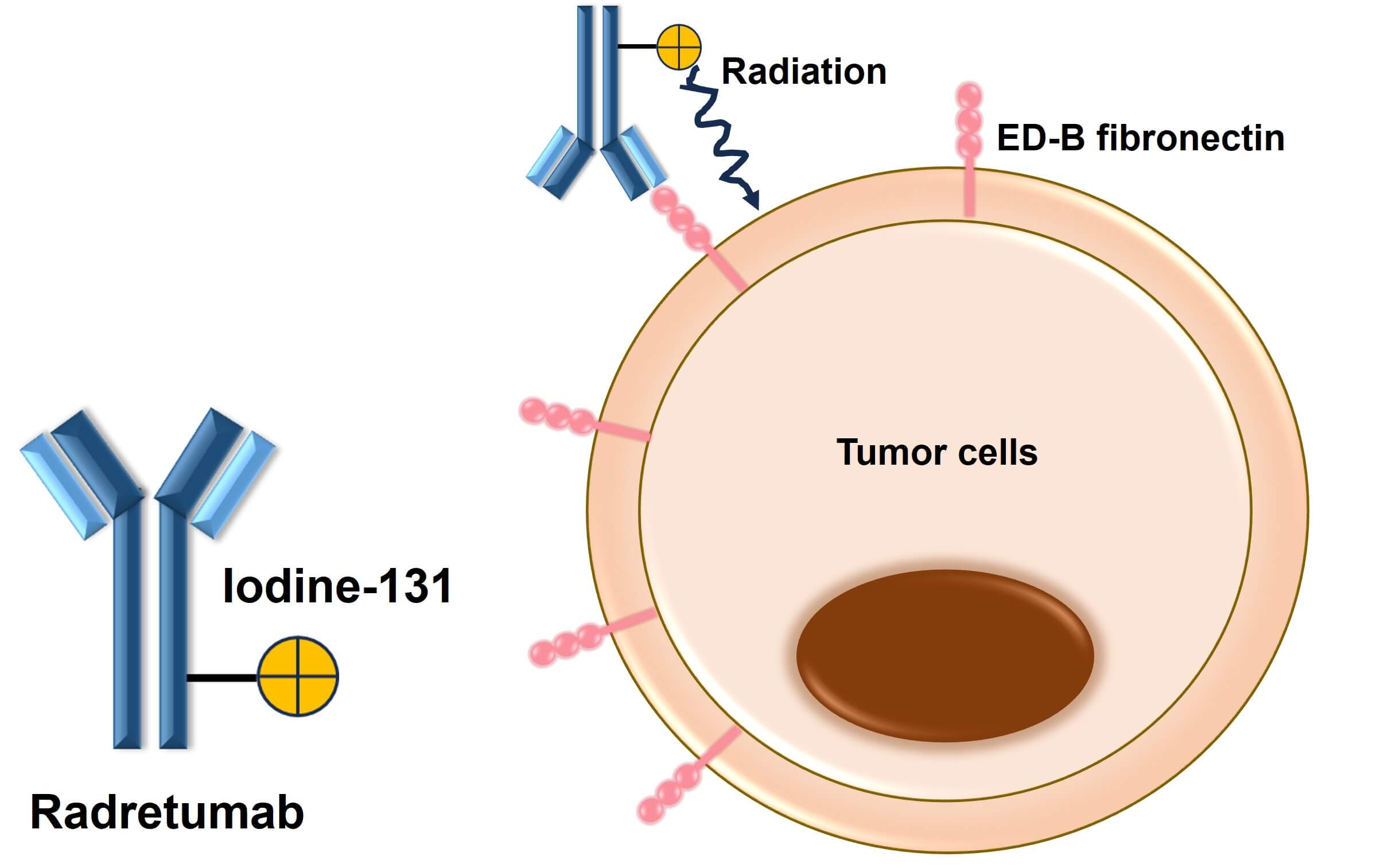 Figure 2. The Mechanism of Radretumab Action (Creative Biolabs Original)
Figure 2. The Mechanism of Radretumab Action (Creative Biolabs Original)
The Clinical Applications of Radretumab
Radretumab's precise targeting mechanism offers several clinical applications:
1. Cancer Therapy:
- Monotherapy: Directly inhibits tumor growth by binding to ED-B fibronectin, hampering angiogenesis and initiating immune responses.
- Combination Therapy: Used with other cytotoxic agents or immune checkpoint inhibitors to enhance therapeutic outcomes.
- Radioimmunotherapy: Conjugated with radioisotopes to deliver focal radiation to tumors.
- Theragnostic: Used radretumab labeled with imaging agents for targeted tumor imaging followed by therapeutic intervention.
2. Diagnostic Imaging:
- Radretumab conjugated with imaging agents can serve as a diagnostic tool to localize tumors and assess the expression of ED-B fibronectin, aiding in precision diagnostics.
3. Targeted Drug Delivery:
- Radretumab can act as a carrier for cytotoxic drugs, ensuring these drugs are delivered specifically to tumor cells, thus sparing healthy tissue and reducing side effects.
Clinical Projects of Radretumab*
| NCT ID | Study Title | Study Status | Conditions | Sponsor | Start Date |
| NCT01125085 | 131I-L19SIP Radioimmunotherapy (RIT) in Combination With External Beam Radiation in Patients With Multiple Brain Metastases From Solid Tumors | COMPLETED | Brain Metastases From Solid Tumors | Philogen S.p.A. | 2009-10 |
* The table is excerpted from the following website: https://clinicaltrials.gov/search?cond=131I-L19SIP
What We Provide
Anti-Human Fibronectin Extra Domain-B Recombinant Antibody (Radretumab)
We offer superior radretumab for utilization in ELISA, IP, FC, FuncS, Neut, IF, ICC and most other immunological methods. The antibody is intended for lab research use only, not for diagnostic, therapeutic, or any in vivo human use.
- Immunogen
- A region in the ED-A domain of human cellular fibronectin.
- Host Species
- Human
- Derivation
- Human
- Type
- [(scFv - heavy - kappa) - IGHE - CH4]2
- Specificity
- Tested positive against native human antigen.
- Species Reactivity
- Human
- Applications
- Suitable for use in ELISA, IP, FC, FuncS, Neut, IF, ICC and most other immunological methods.
- CAS
- 1253180-81-2
- Generic Name
- Radretumab
- Related Disease
- Solid tumors
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.



