Ranibizumab Overview
Introduction of Ranibizumab
Ranibizumab is a monoclonal antibody fragment (Fab) created from the same parent mouse antibody as bevacizumab. Ranibizumab is used to treat wet age-related macular degeneration (AMD; an ongoing disease of the eye that causes loss of the ability to see straight ahead and may make it more difficult to read, drive, or perform other daily activities). It is also used to treat macular edema after retinal vein occlusion (an eye disease caused by blockage of blood flow from the eye that leads to blurry vision and vision loss), diabetic macular edema (an eye disease caused by diabetes that can lead to vision loss), and diabetic retinopathy (damage to the eyes caused by diabetes). It inhibits angiogenesis by inhibiting Vascular endothelial growth factor A (VEGF-A), and its effectiveness is similar to that of bevacizumab. Its rates of side effects also appear similar.
Mechanism of Action of Ranibizumab
Neovascular (“wet”) age-related macular degeneration (AMD) is a pathologic age-related condition of the macula causing severe impairment of vision in people aged above 50 years. Neovascular AMD is characterized by central vision loss due to exudation, hemorrhage, and fibrovascular tissue formation secondary to pathologic choroidal neovascularization (CNV). Factors presumed to be involved in pathogenesis of CNV include ischemia, inflammation, and secretion of angiogenic factors, such as vascular endothelial growth factor (VEGF), which is a homodimeric glycoprotein that has been isolated from CNV membranes obtained from patients with AMD. VEGF belongs to a family of dimeric glycoproteins within the superfamily of PDGFs. While VEGF, also known as VEGF-A, is the most comprehensively studied, other members of this family include VEGF-B, VEGF-C, VEGF-D, and PLGF. VEGF-A has several isoforms that arise from alternative splicing. All VEGF ligands bind to tyrosine kinase receptors, causing the receptors to dimerize and autophosphorylate. Upon binding to its receptor, VEGF initiates a cascade of signaling events that begins with auto-phosphorylation of both receptor kinases, followed by activation of numerous downstream proteins, including phosphoinositide-3-kinase (PI3K), the Ras GTPase activating protein, Ras, mitogenactivated protein kinase (MAPK), and others. VEGF-A binds to VEGF receptor (VEGFR)-1 and VEGFR-2. VEGF promotes the growth, migration, and proliferation of endothelial cells. In addition, VEGF induces vasodilatation and enhances endothelial cell survival. These biologic activities occur in few physiologic processes outside wound healing and ovulation, making VEGF an attractive target for therapy. Ranibizumab binds to VEGF-A, and thereby inhibits the binding of VEGF-A to its receptors, neutralizing the biological activity of VEGF-A and regressing the vascularization, thereby inhibiting the AMD process.
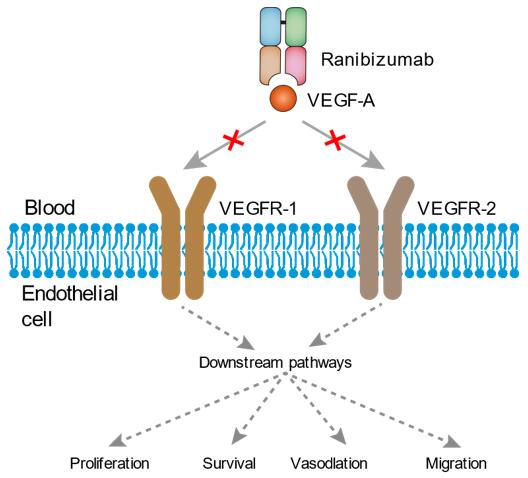 Fig.1 Mechanism of action of Ranibizumab
Fig.1 Mechanism of action of Ranibizumab
What We Provide
Therapeutic Antibody
Ranibizumab
We provide high-quality Ranibizumab for use in WB, FC, IP, ELISA, Neut, FuncS, IF and most other immunological methods. For lab research use only, not for diagnostic, therapeutic or any in vivo human use.
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.




