Rontalizumab Overview
Introduction of Rontalizumab
Rontalizumab is a humanized IgG1 type monoclonal antibody directed against interferon α (IFN-α), a cytokine with potent immunomodulatory properties. This drug has been investigated in the clinical trial for the treatment systemic lupus erythematosus (SLE), a chronic autoimmune disease characterized by the presence of autoantibodies and inflammation in multiple organ systems. A phase I trial on the safety and pharmacokinetics of rontalizumab was conducted in adult patients with mildly active systemic lupus erythematosus. This study investigated the impact of rontalizumab on the expression of IRGs, IFN-inducible proteins, and autoantibodies through detecting the rontalizumab’s safety, tolerability, pharmacokinetics (PK), and pharmacodynamics (PD). And the result supports further evaluation of rontalizumab safety and efficacy in systemic lupus erythematosus. Then, based on the acceptable safety and pharmacodynamic effects in a Phase I study, a Phase I trial to examine the safety and efficacy of rontalizumab was conducted in 2016. Although rontalizumab was safe and well tolerated in patients with active extrarenal lupus, this study did not achieve its primary efficacy endpoint. In addition, a structural study of rontalizumab was conducted to investigate the structural basis of it interacts with IFN-α.
Mechanism of Action of Rontalizumab
Rontalizumab is designed to bind to most of the IFN-α subtype, not IFN-β or IFN-ω, with high affinities. IFN-α is a type I interferon factor which is essential to a vast array of immunologic processes.
Type I interferons consist of a large number of interferon proteins that defense against viral infections and help regulate the activity of the immune system. By binding to type I IFN receptor (IFNAR), type I interferons trigger several signal transduction pathways and play important roles in multiple pathological and physiological processes. The receptor of all type I interferons is a specific cell surface protein complex known as the IFN-α receptor (IFNAR) which is composed by IFNAR1 and IFNAR2 chains. Canonical type I IFN signaling is mediated by Janus kinase (JAK)–signal transducer and activator of transcription (STAT) pathway. Type I IFN signal leads to the transcription of IFN-stimulated genes (ISGs), regulating a number of cellular functions. Type I interferons are secreted by infected cells and have three major functions, including inducing cell-intrinsic antimicrobial states in infected and neighbouring cells, promotes antigen presentation and natural killer cell functions while restraining pro-inflammatory pathways and cytokine production, activate the adaptive immune system. Type I interferons have been demonstrated to involve in diseases associated with chronic infection, autoimmunity and virus infection.
IFN-α is one of the eight cytokines of type I interferon subfamily. It is a cytokine of 188 amino acids mainly involved in an innate immune response against viral infection. It is contributed to orchestrate subsequent signal pathways in innate and adaptive immunity and is mainly employed as a standard therapy for a number of tumors and viral infections. Disease associated with IFN-α including autoimmune thyroid disease, systemic lupus erythematosus, rheumatoid arthritis, primary biliary cholangitis, and type 1 diabetes. The mechanism of action of rontalizumab is to block the signal transduction of type I IFN receptor via neutralizing all 12 IFN-α subtypes.
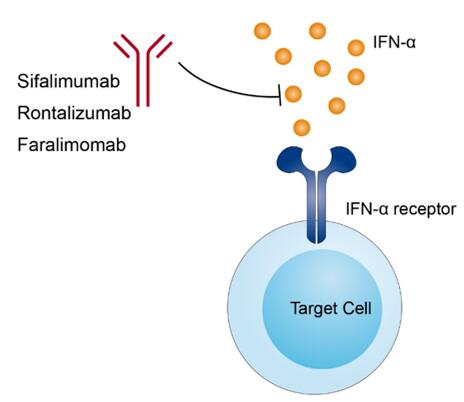 Fig.1 Mechanism of action of Rontalizumab
Fig.1 Mechanism of action of Rontalizumab
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

