Tamtuvetmab Overview
Introduction of Tamtuvetmab
Tamtuvetmab, also known as AT-005 or Tactress®, is a recombinant monoclonal antibody that targets CD52 present in immune cells. Developed by Aratana Therapeutics, Inc., this mAb received veterinary drug approval for treating naïve T-cell lymphoma in dogs from the United States Department of Agriculture (USDA) in January 2014. In a clinical trial evaluating tamtuvetmab for treating canine peripheral nodal T-cell lymphoma, dogs were randomized to receive either a placebo or tamtuvetmab alongside a 19-week L-CHOP chemotherapy protocol. The median progression-free survival (PFS) was 103 days in the placebo group and 64 days in the tamtuvetmab group. Despite the shorter PFS in the treatment group, the overall response rate (ORR) was high, reaching 98% across both groups. Complete response rates were 67% for dogs receiving tamtuvetmab and 64% for the placebo group, showing that while tamtuvetmab did not significantly extend PFS, it demonstrated strong overall efficacy when combined with chemotherapy. In summary, tamtuvetmab has shown significant in vivo efficacy in clinical trials and is used as an antineoplastic treatment in veterinary oncology. However, it is associated with some adverse effects like vomiting, weight loss, diarrhea, and lethargy.
The stated target, CD52, is a small glycoprotein widely expressed on the surface of various immune cells, including lymphocytes, monocytes, and eosinophils. It plays a significant role in immune system regulation and is a target for therapeutic interventions, especially in hematological conditions. By targeting CD52, these therapies induce cell lysis via complement-mediated cytotoxicity and antibody-dependent cell-mediated cytotoxicity (ADCC), leading to immunosuppression.
Biological and Chemical Properties of Tamtuvetmab
Protein Structure of CD52
 Figure 1. The Structure of Human CD52 (UniProt)1,2
Figure 1. The Structure of Human CD52 (UniProt)1,2
The Mechanism of Tamtuvetmab Action
Tamtuvetmab specifically targets the CD52 antigen expressed on various types of immune cells. CD52 is a small glycoprotein anchored to the cell membrane via a glycosylphosphatidylinositol (GPI) link. It is widely expressed on the surface of mature lymphocytes (both B and T cells), monocytes, macrophages, eosinophils, and natural killer (NK) cells. While CD52 is highly expressed on most immune cells, it is absent on hematopoietic stem cells, which allows for therapeutic interventions without affecting bone marrow regeneration. Under normal physiological conditions, CD52 does not have an established role in signaling. However, its widespread presence on immune cells makes it an essential marker for targeted therapies to deplete immune cell populations. In conditions such as autoimmune disorders or cancers like chronic lymphocytic leukemia (CLL), targeting CD52 allows for the selective depletion of immune cells that contribute to disease pathology. Particularly, CD52-targeted antibodies (such as tamtuvetmab) can eliminate these cells through antibody-dependent cell-mediated cytotoxicity (ADCC), complement-dependent cytotoxicity (CDC), and direct apoptosis, helping control aberrant immune responses or reduce cancer cells.
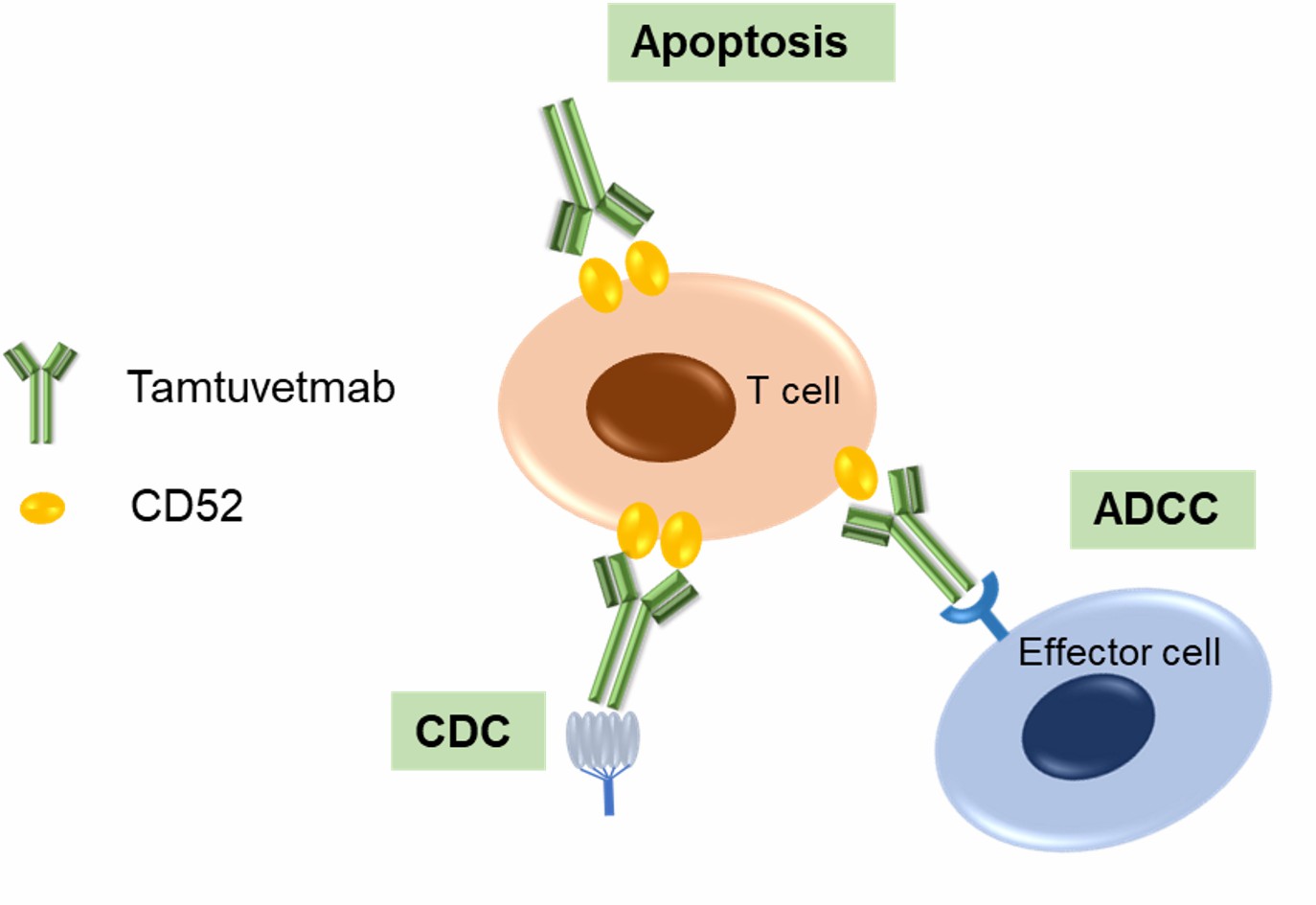 Figure 2. The Mechanism of Tamtuvetmab Action (Creative Biolabs Original)
Figure 2. The Mechanism of Tamtuvetmab Action (Creative Biolabs Original)
The Clinical Applications of Tamtuvetmab
Tamtuvetmab is primarily applied in the treatment of canine T-cell lymphoma, one of the most common cancers in dogs accounting for approximately 15-20% of all lymphomas. This malignancy is highly abundant T cells, and tamtuvetmab has shown great efficacy in treating it. In clinical trials conducted on dogs with T-cell lymphoma, the results demonstrated that tamtuvetmab, when combined with chemotherapy, achieved a high overall response rate (ORR) of 98%, leading to a 30-40% reduction in tumor volume in several treated animals.
Aside from its anti-cancer effects, tamtuvetmab's immunomodulatory properties allow it to be explored for other immune-related diseases. CD52 is expressed on a broad range of immune cells, including T cells and B cells. By depleting these populations, tamtuvetmab has potential utility in autoimmune diseases. In veterinary research, similar anti-CD52 therapies have shown promise in mitigating autoimmune disorders where aberrant immune activity is the central problem. For example, in autoimmune hemolytic anemia (AIHA) and immune-mediated thrombocytopenia (IMT), depleting CD52-positive cells can help reset the immune system, though this application is still under clinical investigation.
In oncology, tamtuvetmab offers a novel treatment option for canine lymphomas, particularly in chemotherapy-resistant cases. Its ability to reduce tumor burden provides veterinarians with a more targeted immunotherapy. In addition to veterinary oncology, the growing understanding of its mechanisms opens the door to broader clinical applications, particularly in managing immune dysregulation disorders.
What We Provide
Anti-Canine CD52 Recombinant Antibody (Tamtuvetmab)
We provide high-quality tamtuvetmab for use in immunoassay techniques such as Enzyme-Linked Immunosorbent Assay, Immunohistochemistry, Immunofluorescence, Immunoprecipitation, Flow Cytometry, and Functional Studies. The product is for lab research use only, not for diagnostic, therapeutic, or any in vivo human use.
- Immunogen
- The details of the immunogen for this antibody are not available.
- Host Species
- Canine
- Derivation
- Chimeric (Dog/Rat)
- Type
- IgG2, λ
- Specificity
- Canine CAMPATH-1 antigen
- Species Reactivity
- Canine
- Applications
- Used for immunoassay techniques such as: Enzyme-Linked Immunosorbent Assay; Immunohistochemistry; Immunofluorescence; Immunoprecipitation; Flow Cytometry; Functional Studies
- Conjugate
- Unconjugated
- Trade name
- TACTRESS
- CAS
- 1608122-71-9
- Generic Name
- Tamtuvetmab
- UNII
- 03E1FV7JUC
- Related Disease
- Veterinary antineoplastic
- UniProt Database (https://www.uniprot.org/uniprotkb/Q28896/entry#structure)
- The image was retrieved from UniProt Database and used under [CC BY 4.0] without modification.
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.



