Tremelimumab Overview
Introduction of Tremelimumab
Tremelimumab (CP-675,206; previously, ticilimumab) is a fully human IgG2 monoclonal antibody and has a half-life of 22 days, that has a subnanomolar affinity for binding to human cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) for treatment of patients with advanced cancers. Treatment with an anti-CTLA4 mAb prevents normal downregulation of T cells and prolongs T cell activation, thereby enhancing immune function. Tremelimumab is under investigation for the treatment of Mesothelioma, Liver Cancer, Liver Neoplasms, Liver Cell Caricinoma, and HepatoCellular Carcinoma and it has been investigated in Part C: Malignant Mesothelioma and Part A and B: Advanced Solid Malignancies. There was no statistically significant difference between treated patients and placebo patients, and side effects such as diarrhoea, loss of appetite and development of rashes were seen at higher rates in treated patients than in placebo treatments. This indicates a need for further research into the use of tremelimumab as a monotherapy as well as use in combination therapy to potentially achieve better clinical outcomes.
Mechanism of Action of Tremelimumab
T lymphocytes can recognize and destroy tumor cells. Full T cell activation requires engagement of T cell receptor(TCR) to antigen-bound major histocompatibility complex(MHC) on antigen presenting cells (APCs) as well as engagement of CD28 on the T cell surface by members of the B7 family (e.g., B7.1/CD80, B7.2/CD86, B7-H3, B7-H4) on APCs. However, an inhibitory mechanism interrupts this destruction. CTLA-4, a homology of CD28, is an activation-induced, type I transmembrane protein of the Ig superfamily, expressed by T lymphocytes as a covalent homodimer that has a higher binding affinity than CD28 for B7. Crosslinking of CTLA-4 by B7 in the context of T-cell antigen receptor (TCR) engagement inhibits T-cell activation, IL-2 gene transcription, and T-cell proliferation by directly inhibiting TCR signal transduction. Thus, CD28-B7 singnal pathway, the second activating signal, is blocked and results in overall downregulation of T cell activation. Immunotherapy is an emerging therapy that has acquired clinical interest in cancer treatment. The use of monoclonal antibodies (mAbs) in cancer has shown good promise. MAbs interfere with a single target molecule, with high selectivity due to their specificity. Tremelimumab, a fully human IgG2 monoclonal antibody, was designed to bind CTLA4 and blocked its interaction with B7 on APCs, which can prevent CD28-B7 down regulatory signal. Without the CTLA4-B7 down regulatory signal, T cell function is enhanced and prolonged, as measured by increased production of IL-2, IFN-γ, IL-3, IL-4, IL-5 and IL-10, thus allows the lymphocytes to continue to destroy cancer cells. Generally, tremelimumab binds to CTLA-4, blocking the inhibitory signal, which prolongs T-cell activation and allows the T cells to destroy the tumor cells.
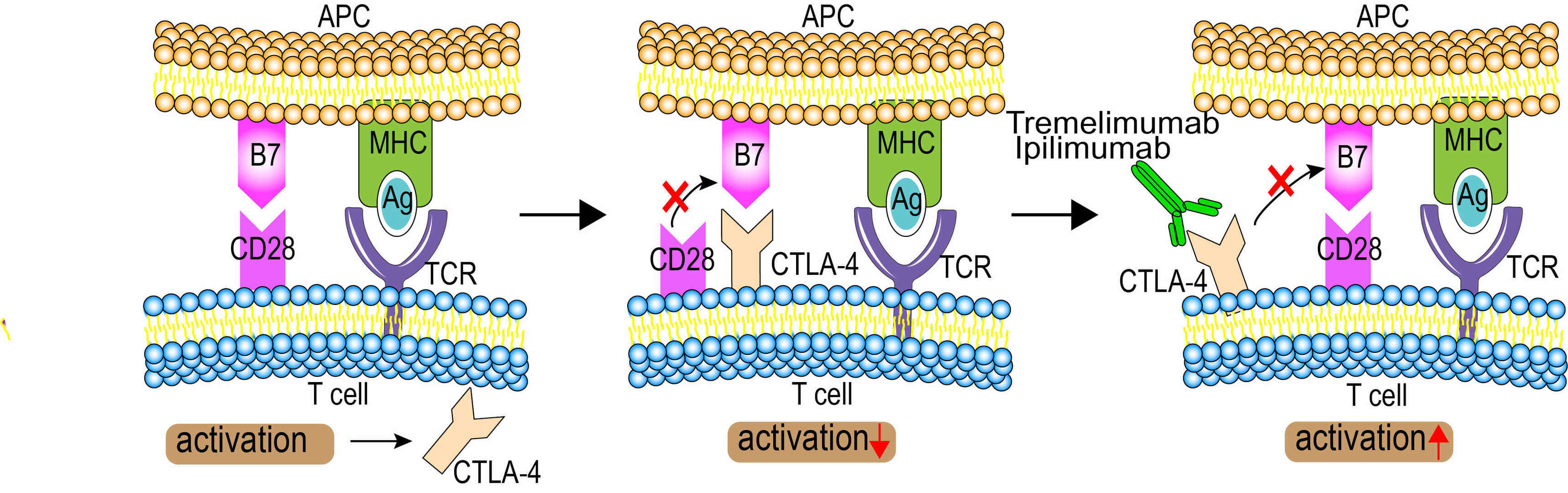 Figure 1 Mechanism of Action of Tremelimumab
Figure 1 Mechanism of Action of Tremelimumab
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.



