Narnatumab Overview
Introduction of Narnatumab
Narnatumab, also known as ABT-700 or IMC-RON8, is a fully human monoclonal antibody that has shown promise in targeting the Ron (Recepteur d'Origine Nantais) tyrosine kinase receptor, a critical regulator of cellular processes involved in cancer progression. As an innovative therapeutic agent initially developed by ImClone Systems, Narnatumab was being explored for its potential in treating various cancers by specifically inhibiting Ron receptor-mediated signaling pathways. However, its clinical development was abandoned after phase I trials.
The Ron Tyrosine Kinase Receptor and Its Role in Cancer
Functionality of Ron
The Ron receptor (MST1R) is a member of the macrophage-stimulating receptor (MSR) family of tyrosine kinases and is primarily involved in regulating cellular responses to injury, infection, and immune response. In normal cells, Ron is activated when the ligand, macrophage-stimulating protein (MSP), binds to the receptor, then leading to receptor dimerization and subsequent activation of its intrinsic tyrosine kinase activity. This activation triggers several downstream signaling cascades, including the PI3K/AKT and MAPK/ERK pathways, which are crucial for regulating cell survival, proliferation, migration, and angiogenesis.
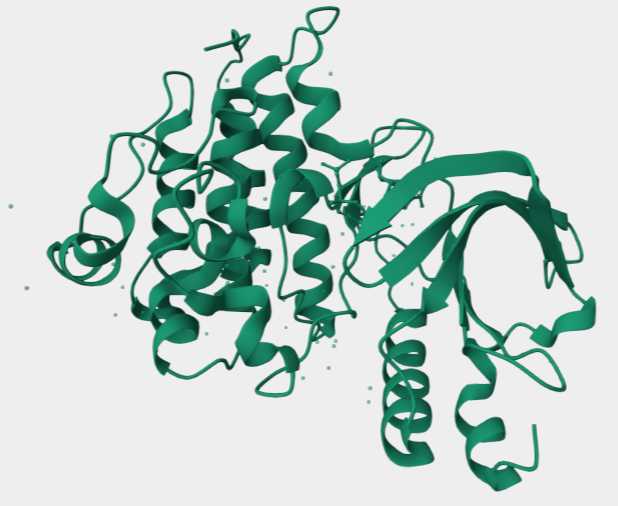 Figure 1. The Structure of Human RON1,2.
Figure 1. The Structure of Human RON1,2.
However, in cancers, the overexpression or aberrant activation of Ron has been implicated in the malignant transformation of cells, which is strongly associated with enhanced tumor growth, metastasis, and resistance to conventional therapies. Ron's ability to activate pro-survival pathways and alter the tumor microenvironment contributes to immune evasion, making it an attractive therapeutic target.
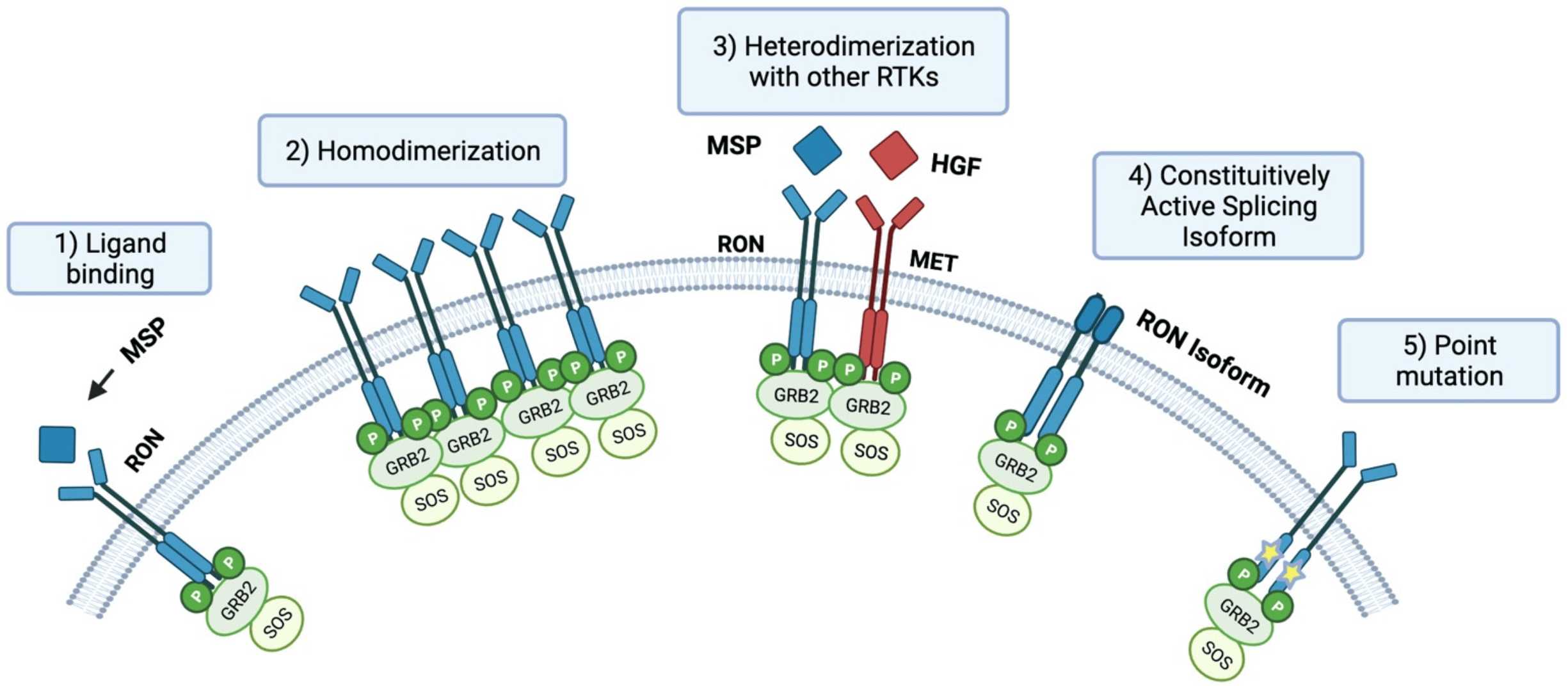 Figure 2. RON Activation Process3.
Figure 2. RON Activation Process3.
The Importance of Targeting Ron in Cancers
The role of Ron in various cancers has been well documented, and its expression is often elevated in many tumor types, including non-small cell lung cancer (NSCLC), ovarian cancer, and gastric cancer. These cancers are typically associated with poor prognosis, and their resistance to standard therapies makes them ideal candidates for targeted treatments. By targeting the Ron receptor with monoclonal antibodies like Narnatumab, it becomes possible to block the signaling pathways that contribute to tumor progression and metastasis.
Biological and Chemical Properties of Narnatumab
Protein Chemical Formula
C6454H10026N1754O2020S44
Protein Average Weight
The molar mass of the protein is approximately 145922.10 g/mol.
Mechanism of Action of Narnatumab in Targeting Ron
Narnatumab is designed to specifically bind to the extracellular domain of the Ron receptor, preventing its activation. Upon binding, Narnatumab effectively inhibits the dimerization of Ron, a crucial step required for the receptor's activation. When two Ron receptors bind together in response to MSP, dimerization occurs, which leads to autophosphorylation on specific tyrosine residues. This phosphorylation is essential for initiating downstream signaling that promotes tumor cell survival, migration, and angiogenesis. By blocking the dimerization and subsequent phosphorylation of Ron, Narnatumab disrupts the signaling cascade, which results in reduced tumor cell proliferation, migration, and survival. In essence, Narnatumab serves as a competitive inhibitor of Ron activation, directly influencing cancer cell behavior and preventing the receptor from driving tumor progression.
Furthermore, by blocking Ron signaling, Narnatumab can reduce angiogenesis, thus limiting tumor growth and metastasis. Angiogenesis is the formation of new blood vessels from pre-existing ones, which tumors exploit to supply themselves with oxygen and nutrients. This vascular network supports not only the primary tumor's growth but also facilitates the spread of cancer cells to distant sites through the bloodstream. By inhibiting Ron signaling, Narnatumab disrupts this critical angiogenic process, effectively starving the tumor of its blood supply and preventing the establishment of metastases. This reduction in angiogenesis is a key therapeutic effect of Narnatumab, as it targets both tumor growth and the ability of cancer cells to spread.
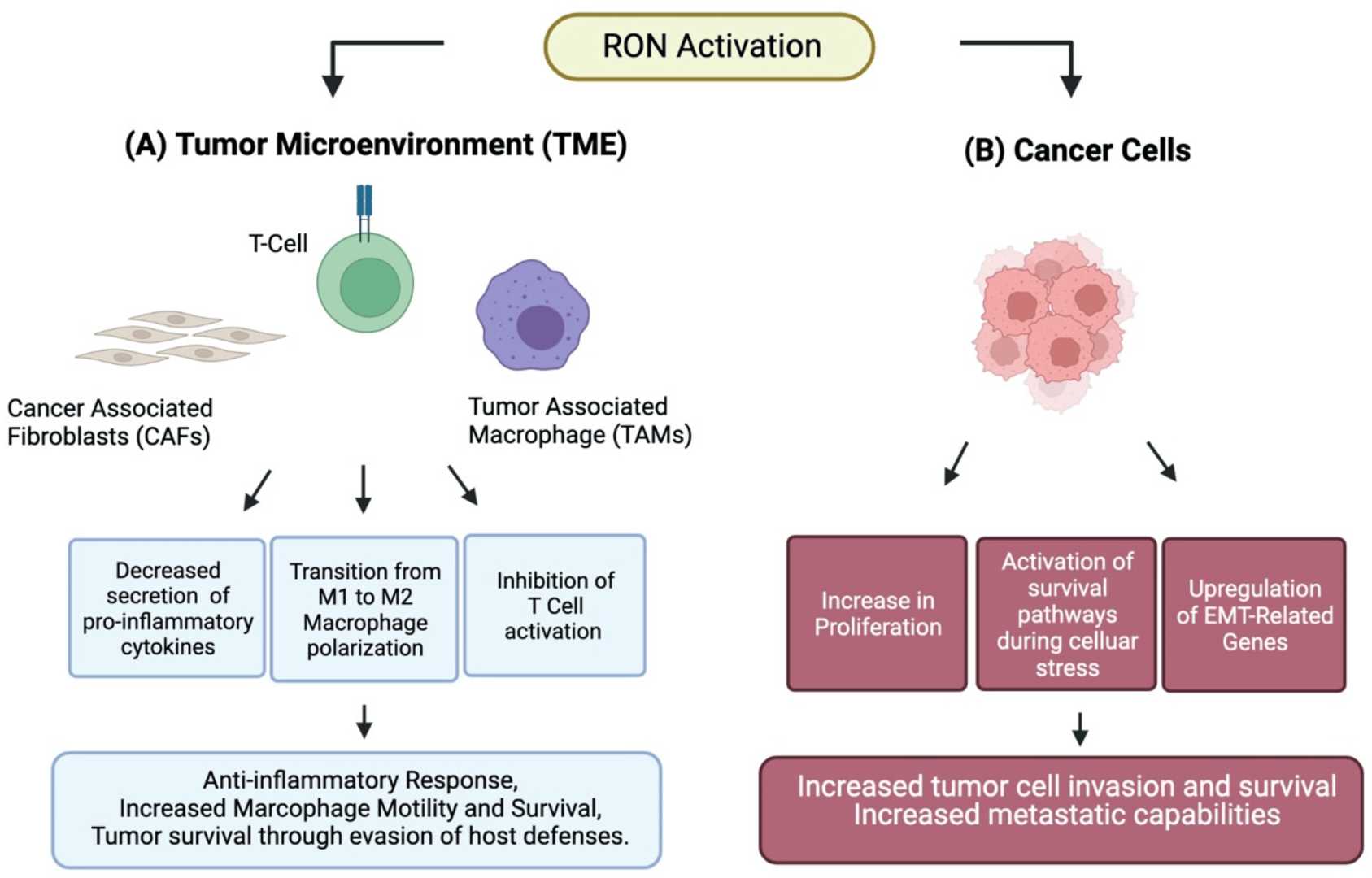 Figure 3. RON Activation in Tumors and in TME3.
Figure 3. RON Activation in Tumors and in TME3.
Clinical Applications of Narnatumab
Preclinical and Clinical Evidence
The therapeutic potential of Narnatumab has been evaluated in a variety of preclinical models. In studies involving human cancer xenografts, Narnatumab has demonstrated significant tumor growth inhibition, particularly in cancers known to overexpress Ron. For instance, in models of non-small cell lung cancer (NSCLC), treatment with Narnatumab resulted in reduced tumor volume and improved survival rates. Similarly, in ovarian and gastric cancer models, Narnatumab exhibited potent anti-tumor activity by inhibiting both primary tumor growth and metastatic spread.
In clinical trials, Narnatumab has shown a promising safety and efficacy profile, particularly in patients with cancers that exhibit elevated Ron expression. Early-phase trials have reported manageable side effects, which are typical of monoclonal antibody treatments, including mild infusion reactions and gastrointestinal symptoms. Moreover, partial responses have been observed in patients treated with Narnatumab, especially when used in combination with other therapeutic modalities.
Combination Therapy with Narnatumab
One of the most exciting aspects of Narnatumab's therapeutic potential lies in its ability to enhance the efficacy of other cancer treatments. Combination therapies are often more effective in overcoming the complex mechanisms of tumor resistance, and Narnatumab has shown promising synergistic effects when combined with chemotherapy or immune checkpoint inhibitors.
- Chemotherapy: Narnatumab has been found to sensitize cancer cells to chemotherapy agents, such as paclitaxel and cisplatin, by inhibiting the pro-survival PI3K/AKT and MAPK/ERK pathways. In preclinical models, Narnatumab combined with chemotherapy resulted in enhanced tumor regression compared to chemotherapy alone.
- Immune Checkpoint Inhibitors: The tumor microenvironment is a critical barrier to the success of immune checkpoint inhibitors, which block inhibitory signals to enhance immune cell activity. Narnatumab's ability to modulate the TME and reduce immune suppression makes it a valuable candidate for combination with immune checkpoint inhibitors, such as pembrolizumab. By improving immune cell function and increasing tumor antigen presentation, Narnatumab may boost the effectiveness of these immunotherapies.
Clinical Projects of Narnatumab*
| NCT ID | Study Title | Study Status | Conditions | Sponsor | Start Date |
| NCT01472016 | Study of ABT-700 in Subjects With Advanced Solid Tumors | COMPLETED | Advanced Solid Tumors | AbbVie (prior sponsor, Abbott) | 2011/10/6 |
* The table was excerpted from the following website: https://clinicaltrials.gov/search?cond=ABT-700
What We Provide
Anti-Human RON Recombinant Antibody (Narnatumab)
We provide high-quality narnatumab for use in IF, IP, Neut, FuncS, ELISA, FC, ICC, and most other immunological methods. The product is for lab research use only, not for diagnostic, therapeutic, or any in vivo human use.
- Immunogen
- RE7 cells and MDCK cells overexpressing the human RON receptor in complete Freund`s adjuvant
- Host Species
- Human
- Derivation
- Human
- Type
- IgG1 - kappa
- Specificity
- Tested positive against native human antigen.
- Species Reactivity
- Human
- Applications
- Suitable for use in IF, IP, Neut, FuncS, ELISA, FC, ICC and most other immunological methods.
- CAS
- 1188275-92-4
- Generic Name
- Narnatumab
- MW
- 145.9 kDa
- Related Disease
- Solid tumors
- UniProt Database (https://www.uniprot.org/uniprotkb/Q04912/entry#structure)
- The image was retrieved from UniProt Database and used under [CC BY 4.0] without modification.
- Cazes, Alex, et al. "The MST1R/RON tyrosine kinase in cancer: oncogenic functions and therapeutic strategies." Cancers 14.8 (2022): 2037. Distributed under Open Access license CC BY 4.0, without modification.
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.



