Tovetumab Overview
Introduction of Tovetumab
Tovetumab, a human monoclonal antibody, represents an innovative approach to cancer treatment by targeting PDGFRA. This tyrosine kinase receptor has drawn significant interest due to its role in tumor growth. Research indicates that PDGFRA is particularly active in aggressive cancers, notably glioblastoma and non-small cell lung cancer (NSCLC). AstraZeneca developed Tovetumab and conducted several clinical trials to evaluate its potential. A Phase I open-label study tested various doses to assess safety and effectiveness in patients with advanced solid tumors. While the drug was found to be safe for intravenous use, it showed minimal impact on tumor growth. Additional trials yielded similarly disappointing results. By 2013, AstraZeneca ended the development program due to its poor clinical performance. Today, Creative Biolabs provides Tovetumab solely for research use, helping scientists better understand PDGFRA-targeted treatments.
Biological and Chemical Properties of Tovetumab
Target Protein Structure
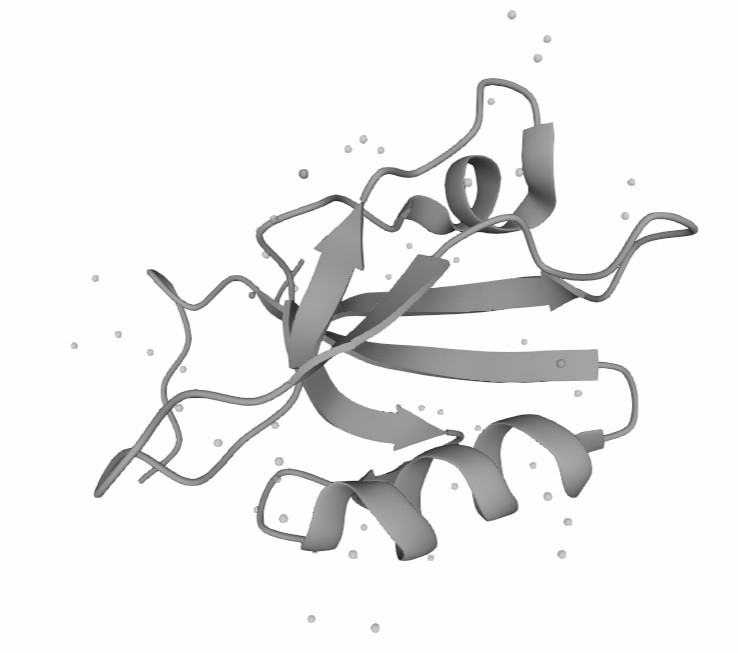 Figure 1. The Structure of Human PDGFRA. (UniProt)1,2
Figure 1. The Structure of Human PDGFRA. (UniProt)1,2
Protein Chemical Formula
C6400H9906N1726O2002S54
The Mechanism of Tovetumab Action
Tovetumab works by interfering with PDGFRA, a receptor that is involved in cell growth and survival. This receptor appears in high levels in several cancer types, including glioblastoma, non-small cell lung cancer, and gastrointestinal stromal tumors (GISTs). Under normal conditions, PDGFRA helps with tissue repair and growth. However, cancer cells often produce too much of this receptor, leading to uncontrolled tumor growth through pathways like PI3K/AKT and MAPK/ERK. Tovetumab attaches to the outer part of PDGFRA on cancer cells, preventing its dimerization and subsequent autophosphorylation in normal receptor activation. This attachment also blocks crucial growth signals and prevents PDGFRA-mediated signal transduction. By disrupting these signals, Tovetumab aims to slow down cancer growth and reduce tumor survival. This approach seemed promising for cancers with high PDGFRA levels, as it targeted both the tumor itself and its surrounding environment. Creative Biolabs provides high-quality, research-grade Tovetumab for scientists seeking to further investigate its molecular mechanisms.
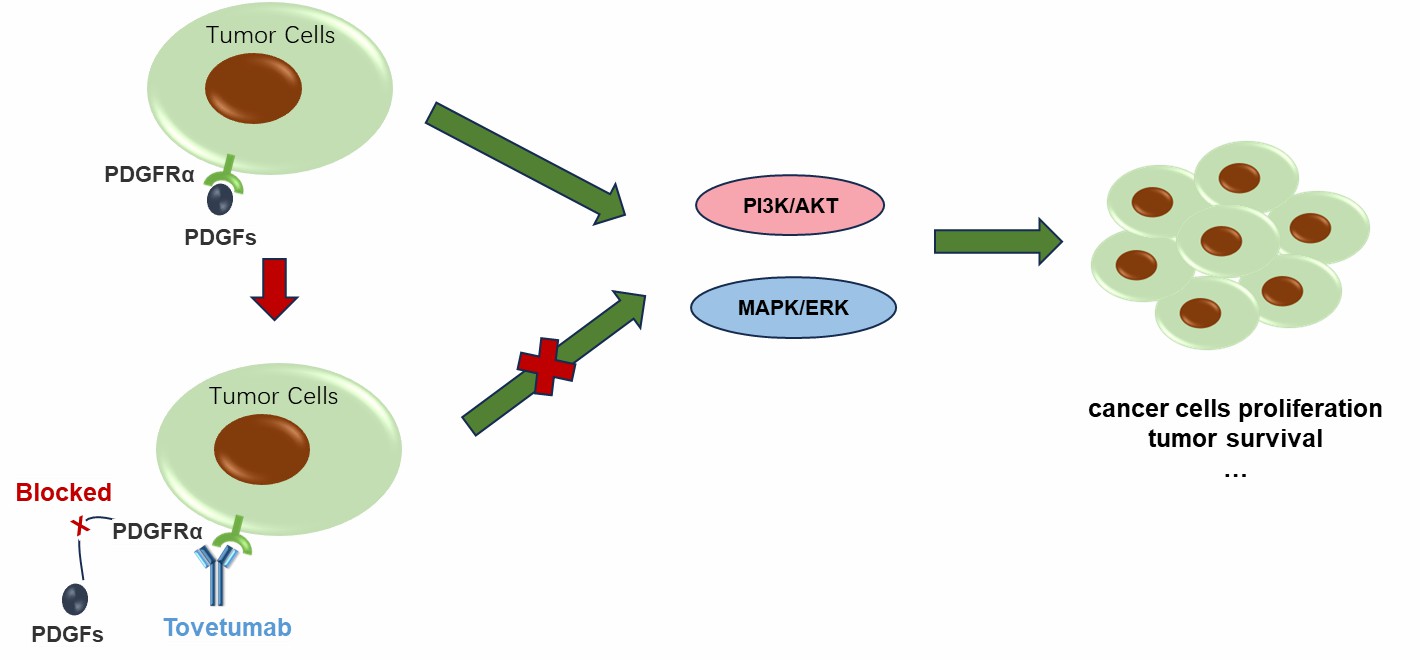 Figure 2. The Mechanism of Tovetumab Action. (Creative Biolabs Original)
Figure 2. The Mechanism of Tovetumab Action. (Creative Biolabs Original)
Clinical Applications of Tovetumab
The potential therapeutic value of Tovetumab lies in its targeted approach against tumors with high PDGFRA expression. This antibody shows promise in treating glioblastoma, non-small cell lung cancer (NSCLC), and gastrointestinal stromal tumors (GIST). Its ability to block PDGFRA disrupts key survival signals in cancer cells, potentially slowing tumor growth and spread. This makes Tovetumab especially relevant for patients whose cancers depend heavily on PDGFRA pathways.
In glioblastoma, where PDGFRA levels are often elevated, Tovetumab's mechanism could weaken the tumor's foundation. It reduces cell growth and the formation of new blood vessels that nourish the tumor. Similarly, in NSCLC, where PDGFRA drives tumor progression and spread, Tovetumab's blocking action might help control the disease, offering hope to patients with limited treatment options. Tovetumab's influence extends beyond targeting the tumor itself. The antibody also affects the tumor's surrounding environment by blocking PDGFRA in nearby support cells. This interference could reduce the tumor's ability to recruit helper cells like fibroblasts and endothelial cells, which are critical for blood vessel formation and nutrient supply. By disrupting this support network, Tovetumab may effectively starve the tumor of the resources it needs to grow.
Given its dual action on both tumor cells and their environment, Tovetumab represents a thoughtful approach to treating PDGFRA-driven cancers. Although its clinical development faced challenges, the scientific principles behind its design remain valuable for cancer research. Creative Biolabs now provides Tovetumab for research use, supporting ongoing efforts to better understand and improve treatments for PDGFRA-related cancers. Continued research may unlock new strategies for targeting similar pathways in cancer treatment.
Clinical Projects of Tovetumab*
| NCT ID | Study Title | Study Status | Conditions | Sponsor | Start Date |
| NCT01102400 | A Study of MEDI-575 in Patients With Advanced Solid Malignancies | Completed | Advanced Solid Malignancies | AstraZeneca | 2010-04-13 |
| NCT01268566 | A Study of MEDI-575 in Subjects With Recurrent Glioblastoma Multiforme | Completed | Glioblastoma Multiforme | MedImmune LLC | 2010-12-31 |
| NCT00816400 | A Dose Escalation, Dose Expansion Study to Evaluate the Safety, Tolerability, and Antitumor Activity of MEDI-575, in Subjects With Advanced Tumors. (MEDI-575) | Completed | Cancer | MedImmune LLC | 2009-01-01 |
* The table was excerpted from the following website: https://clinicaltrials.gov/search?cond=%20MEDI-575
What We Provide
Anti-Human CD140a Recombinant Antibody (Tovetumab)
We provide high-quality tovetumab for use in IP, IF, FuncS, FC, Neut, ELISA, ICC and most other immunological methods. The product is for lab research use only, not for diagnostic, therapeutic, or any in vivo human use.
- Immunogen
- The details of the immunogen for this antibody are not available.
- Host Species
- Human
- Derivation
- Human
- Type
- IgG2 - kappa
- Species Reactivity
- Human
- Applications
- Suitable for use in IP, IF, FuncS, FC, Neut, ELISA, ICC and most other immunological methods.
- CAS
- 1243266-04-7
- Generic Name
- Tovetumab
- MW
- 144.8 kDa
- Related Disease
- Glioblastoma multiforme (GBM)
- UniProt Database (https://www.uniprot.org/uniprotkb/P16234/entry)
- The image was retrieved from UniProt Database and used under [CC BY 4.0] without modification.
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.



