Abituzumab Overview
Introduction of Abituzumab
Abituzumab is a recombinant humanized IgG2 monoclonal antibody targeted at integrin alpha-V (ITGAV), which is a subunit of integrin protein. Abituzumab was primitively developed in an attempt to prevent bone lesion metastases in castration-resistant prostate cancer. Abituzumab has been investigated in clinical trials for the treatment of colorectal cancer, ovarian cancer, prostate cancer, and solid tumors and not approved for any use anywhere in the world.
Recent studies about abituzumab have focused on prostate cancer and colorectal cancer. A Phase I trial of metastatic colorectal cancer has evaluated the safety of tolerability of abituzumab in combination therapy with cetuximab and irinotecan. Although the result did not meet the initial requirements, the over survival in patients receiving abituzumab improved compared to standard of care. Another Phase II clinical trial about the treatment of metastatic castration-resistant prostate cancer has been investigated using abituzumab in combination with luteinizing hormone antagonist/agonist. In addition, a study evaluated the impaction of abituzumab on prostate cancer has conducted by detecting the proliferation, apoptosis, cell cycle, adhesion, detachment, migration, invasion, and phosphorylation of downstream targets, including FAK, Akt, and ERK of prostate cancer cells. Results showed that abituzumab inhibited migration and invasion of prostate cancer cells and decreased phosphorylation of FAK, Akt, and ERK. However, the treatment of prostate cancer with abituzumab still need more investigated.
Mechanism of Action of Abituzumab
The targeted molecular of abituzumab is integrin αv, which could form multiple integrins with different beta units. Integrins are transmembrane receptors that function mechanically, by facilitating the cell cytoskeleton adhesion to the extracellular matrix (ECM), and biochemically, by sensing whether adhesion has occurred. Most integrins bind to its ligands with their extracellular head region and others bind counter-receptors on neighboring cells, bacterial polysaccharides, or viral coat proteins. Integrins transduce biochemical signals into the cell via downstream effector proteins when binds to the extracellular ligands such as fibronectin, vitronectin, collagen, and laminin. The signals mediated by integrins regulate the activities of cytoplasmic kinases, growth factor receptors, and ion channels and control the organization of the intracellular actin cytoskeleton. Previous studies have demonstrated integrins that contain the αv subunit promote the occurrence of malignant tumors, such as melanoma, renal cancer, colorectal cancer, and PCa. Inhibition of αv integrin activation has been shown to reduce cell survival, induce cell cycle blockade, and also reduce tumor growth and metastasis, providing the desired anti-tumor effect for clinical treatment. Based on these findings, the integrin αv subunits has been considered as an effective clinical therapeutic target for tumors.
Abituzumab is designed to target the alpha V subunit, so that it blocks all integrins containing the alpha V chain including alpha V beta 1, alpha V beta 3, alpha V beta 5, alpha V beta 6 and alpha V beta 8. Thus, binding of abituzumab to target αv subunits inhibits the activation of multiple integrin signaling pathways, such as focal adhesion kinase (FAK), AKT, and ERK, which have been shown to promote cell growth, cell motility, cell invasion, and tumor growth. The mechanism of action of abituzumab is recognizing and binding to the αv integrin extracellular domains, blocking the interaction between integrin and its ligands without cross-reacting with other members of the integrin family.
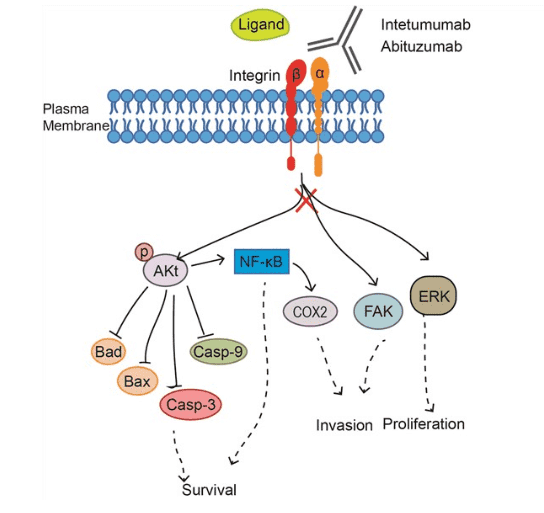 Fig.1 Mechanism of action of Abituzumab
Fig.1 Mechanism of action of Abituzumab
What We Provide
Therapeutic Antibody
Abituzumab
We provide high-quality Abituzumab for use in WB, FC, IP, ELISA, Neut, FuncS, IF and most other immunological methods. For lab research use only, not for diagnostic, therapeutic or any in vivo human use.
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.


