Actoxumab Overview
Introduction of Actoxumab
Actoxumab, recombinant antibody expressed in CHO, is an investigational fully human monoclonal antibody combination for the prevention of Clostridium difficile infection recurrence.
Mechanism of Action of Actoxumab
Clostridium difficile is an anaerobic, spore-forming, Gram-positive bacterium that causes infections in the lumen of the colon and is the most frequent cause of nosocomial diarrhea in the developed world. C. difficile infections (CDI) contribute to thousands of deaths and are associated with over $1 billion in health care-related costs in the United States each year. The symptoms of CDI range from asymptomatic carriage or mild diarrhea to fatal pseudomembranous colitis, colonic rupture, and death. The disease occurs mainly in patients undergoing (or who have recently undergone) a course of broad-spectrum antibiotics; in such patients, composition of the gut microbiota is altered, disrupting the body's natural defense against C. difficile infections. A major concern with CDI is that even when treatment of a primary infection is successful, 20 to 30% of patients experience a recurrence of the disease within days or weeks of symptom resolution. Disease recurrence results from continued disruption of the gut microbiota by standard-of-care antibiotics combined with persistence of resistant C. difficile spores (relapse) or reacquisition of new spores from the environment (reinfection). Multiple recurrences often occur, as repeated antibiotic use prevents the gut microbiota from reestablishing itself, allowing C. difficile spores to germinate and reinfect the gut as soon as antibiotic use is discontinued. These challenges highlight the need for nonantibiotic therapies for CDI that may spare the intestinal microbiota and thus be associated with lower rates of recurrence. The symptoms of CDI are caused by two homologous exotoxins, TcdA and TcdB, expressed by pathogenic strains of C. difficile. The toxins target the epithelial cells of the gut lining by binding to unknown receptors at the cell surface, entering the cells via endocytosis and inactivating Rho-type GTPases through covalent glucosylation. Inactivation of these enzymes leads to dysregulation of the actin cytoskeleton and loss of tight junction integrity, as well as to the release of proinflammatory factors such as interleukin 8 (IL-8). The resulting increase in gut wall permeability and acute proinflammatory response leads to diarrhea and, if left unchecked, to the more severe symptoms of CDI. Actoxumab is a human monoclonal antibody that binds to and neutralizes TcdA. A recent report from a laboratory showed that a novel toxin-neutralizing antibody reverses fulminant CDI in mice when the antibody is given after disease symptoms have developed. It is a viable approach to the treatment and prevention of CDI.
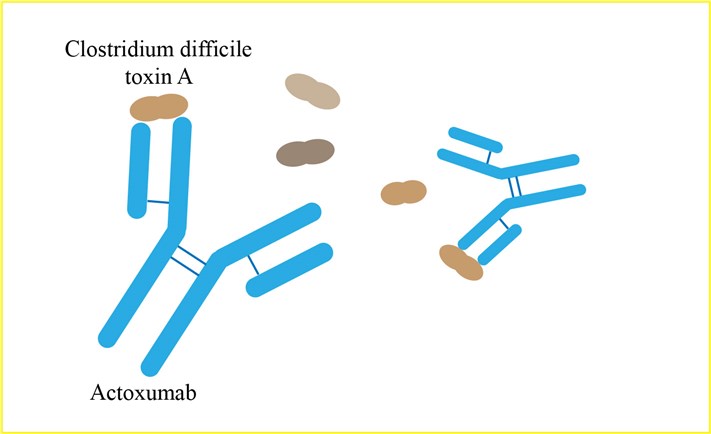 Fig 1. Mechanism of Action of Actoxumab
Fig 1. Mechanism of Action of Actoxumab
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

