Ado-trastuzumab Emtansine Overview
Introduction of Ado-trastuzumab Emtansine
Ado-trastuzumab emtansine is an antibody-drug conjugate (ADC) consisting of the monoclonal antibody (mAb) trastuzumab linked to the cytotoxic agent emtansine (DM1). In the United States, it was approved with the generic name "ado-trastuzumab emtansine", rather than the original United States Adopted Name (USAN) issued in 2009, "trastuzumab emtansine". The "ado-" prefix was added to help prevent dispensing errors. During preclinical development and clinical trials, the drug was also known as trastuzumab-DM1 or trastuzumab-MCC-DM1 (after the codename for emtansine), both abbreviated T-DM1, and by the codename PRO132365. Ado-trastuzumab emtansine was approved in the United States specifically for treatment of HER2-positive metastatic breast cancer (mBC) in patients who have been treated previously with trastuzumab and a taxane (paclitaxel or docetaxel), and who have already been treated for mBC or developed tumor recurrence within six months of adjuvant therapy. Approval was based on the EMILIA study, a phase III clinical trial that compared trastuzumab emtansine versus capecitabine plus lapatinib in 991 people with unresectable, locally advanced or metastatic HER2-positive breast cancer who had previously been treated with trastuzumab and taxane chemotherapy. This trial showed improved progression-free survival in patients treated with trastuzumab emtansine (median 9.6 vs. 6.4 months), along with improved overall survival (median 30.9 vs. 25.1 months) and safety.
Mechanism of Action of Ado-trastuzumab Emtansine
Ado-trastuzumab emtansine is an ideal ADC that combines the best components: specific monoclonal antibody trastuzumab, potent cytotoxic agent DM1, and stable thioether linker. Trastuzumab, a monoclonal antibody targeted to the HER2 receptor, is widely utilized for the treatment of patients with HER2-positive early stage, locally advanced and metastatic breast cancer. This agent causes cell death through antibody-mediated cellular cytotoxicity (ADCC), HER2 downregulation, as well as other proposed mechanisms, and has revolutionized the treatment of HER2-positive breast cancers. DM1 maytansinoid, derivative of maytansine, is the highly potent cytotoxic in trastuzumab emtansine. DM1 is an antimitotic agent that inhibits microtubule assembly leading to cell cycle arrest and apoptosis. This agent is effective at picomolar concentrations. Although DM1 interact with the microtubules as the vinca alkaloid, it is 20-100 times more potent than vincristine. DM1 is also 24-270 times more potent than agents used in conventional chemotherapy, such as paclitaxel. The linker is a nonreducible thioether bond ([N-maleimidomethyl]cyclohexane-1-carboxylate) MCC that binds trastuzumab to DM1. The ideal linker should be stable in circulation to minimize the risk of systemic toxicities. However, the linker should efficiently release the cytotoxic agent upon ADC internalization. After binding to the HER2 receptor, the HER2 receptor and drug-antibody conjugate are internalized via endocytosis. Intracellularly, DM1 is separated from the antibody by lysosomal degradation and binds to tubulin. This disrupts the microtubules causing cell cycle arrest and apoptosis. While trastuzumab is utilized to deliver emtansine to the cell, it also retains its properties of ADCC, inhibition of HER2 signaling pathways, and prevention of HER2 receptor shedding.
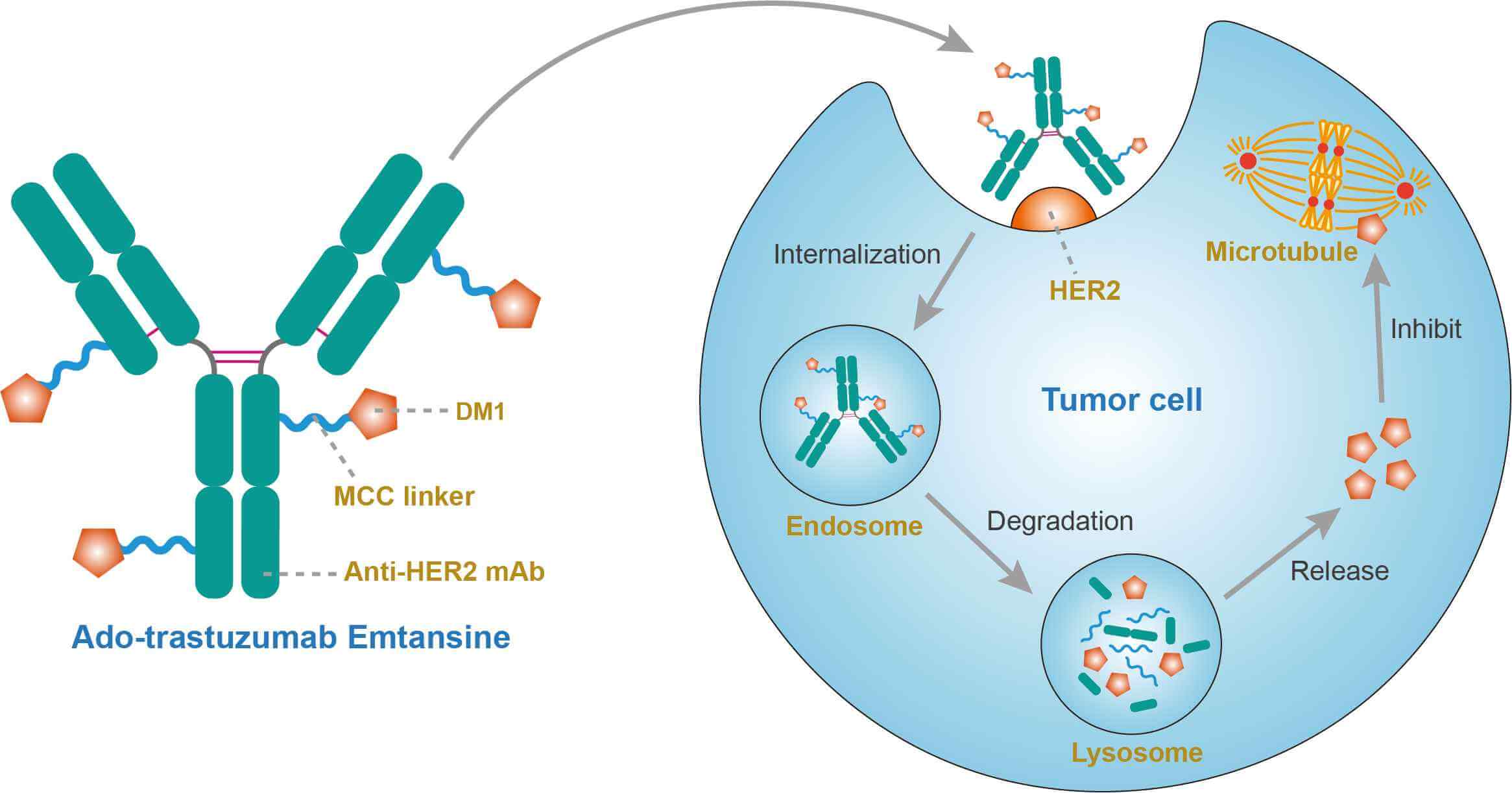 Fig.1 Mechanism of action of ado-trastuzumab emtansine
Fig.1 Mechanism of action of ado-trastuzumab emtansine
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.



