Atezolizumab Overview
Introduction of Atezolizumab
Atezolizumab is an Fc-engineered, humanized, monoclonal antibody (IgG1κ isotype). It was designed to bind to the protein programmed cell death-ligand 1 (PD-L1), which is also known as cluster of differentiation 274 (CD274) or B7 homolog 1 (B7-H1). Atezolizumab has been approved by USA, Canada, Australia, and European Union for the treatment of locally advanced or metastatic non-small cell lung cancer (NSCLC) and metastatic urothelial carcinoma (mUC).
Mechanism of Action of Atezolizumab
PD-L1 is an immune checkpoint molecule expressed on both tumor cells and certain immune cells. The binding of PD-L1 to its receptors PD-1 or B7.1 on activated T cells causes an inhibitory signal to suppress their production in the lymph nodes, thereby preventing immune responses to events such as pregnancy or autoimmune disease. This pathway is also utilized by cancer cells to evade the immune system through evasion of anti-tumor T-cell response. Furthermore, over-expression of PD-L1 and PD-1 has been linked to increased tumor aggressiveness and poorer prognosis. Atezolizumab binds directly and selectively to PD-L1 to block interaction with both PD-1 and B7.1 receptors, thus reinvigorating and enhancing the body’s adaptive anti-cancer activity. Disrupting the PD-L1/B7.1 interaction may also enhance T-cell priming, which could result in increased duration of response and survival.
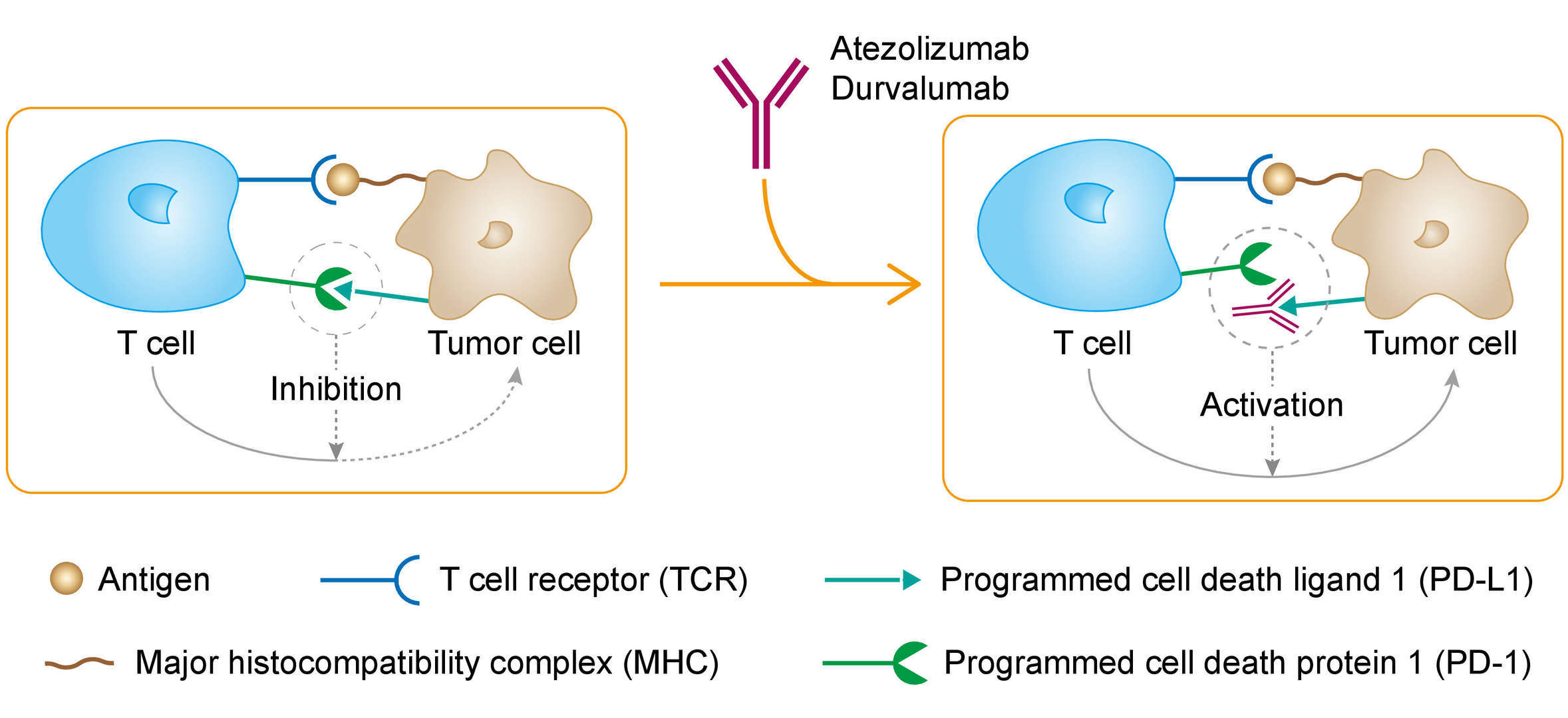 Fig. 1 Mechanism of Action of Atezolizumab
Fig. 1 Mechanism of Action of Atezolizumab
Clinical Projects of Atezolizumab*
| NCT ID | Status | Conditions | Lead Sponsor | Update Time |
| NCT03276468 | Recruiting | Follicular Lymphoma, Diffuse Large B Cell Lymphoma, Marginal Zone Lymphoma, Mucosa Associated Lymphoid Tissue | The Lymphoma Academic Research Organisation | September 8, 2017 |
| NCT03125928 | Recruiting | HER2-positive Breast Cancer | Fox Chase Cancer Center | April 24, 2017 |
| NCT03353831 | Not yet recruiting | Recurrent Ovarian Carcinoma | AGO Research GmbH | November 27, 2017 |
| NCT03120676 | Recruiting | Hodgkin Lymphoma | Memorial Sloan Kettering Cancer Center | April 19, 2017 |
| NCT03359239 | Not yet recruiting | Urothelial/Bladder Cancer, Nos | Matthew Galsky | December 2, 2017 |
| NCT03093922 | Recruiting | Urothelial Carcinoma, Locally Advanced, Unresectable | Memorial Sloan Kettering Cancer Center | March 28, 2017 |
| NCT03181100 | Recruiting | Malignant Neoplasms of Thyroid and Other Endocrine Glands, Anaplastic Thyroid Carcinoma, Poorly Differentiated Thyroid Cancer | M.D. Anderson Cancer Center | June 8, 2017 |
| NCT03074513 | Recruiting | Malignant Neoplasms of Digestive Organs, Lip Oral Cavity and Pharynx, Mesothelial and Soft Tissue, Respiratory and Intrathoracic Organs | M.D. Anderson Cancer Center | March 8, 2017 |
| NCT02989584 | Recruiting | Bladder Cancer, Metastatic Bladder Cancer, Metastatic Bladder Cancer, Urothelial Carcinoma | Memorial Sloan Kettering Cancer Center | December 12, 2016 |
| NCT03272217 | Recruiting | Urothelial Carcinoma | Arjun Balar, MD | September 5, 2017 |
| NCT03108131 | Recruiting | Malignant Neoplasms of Digestive Organs, Melanoma and Other Malignant Neoplasms of Skin, Appendiceal Adenocarcinoma, Cutaneous Squamous Cell Carcinoma, Small Bowel Adenocarcinoma | M.D. Anderson Cancer Center | April 11, 2017 |
| NCT02846623 | Recruiting | Chronic Lymphocytic Leukemia, Small Lymphocytic Lymphoma | M.D. Anderson Cancer Center | July 27, 2016 |
| NCT03174197 | Recruiting | Malignant Neoplasms of Eye Brain and Other Parts of Central Nervous System, Glioblastoma | M.D. Anderson Cancer Center | June 2, 2017 |
| NCT02982694 | Recruiting | MSI, ColoRectal Cancer, Chemotherapy, Resistance, APC | Vall d'Hebron Institute of Oncology | December 5, 2016 |
| NCT02951767 | Active, not recruiting | Bladder Cancer | Hoffmann-La Roche | November 1, 2016 |
| NCT03474094 | Recruiting | Sarcoma,Soft Tissue | Centre Leon Berard | March 22, 2018 |
| NCT03175432 | Recruiting | Melanoma and Other Malignant Neoplasms of Skin | M.D. Anderson Cancer Center | June 5, 2017 |
| NCT02108652 | Active, not recruiting | Bladder Cancer | Hoffmann-La Roche | April 9, 2014 |
| NCT02609984 | Active, not recruiting | Sarcoma, Myxoid/Round Cell Liposarcoma, Synovial Sarcoma, Metastatic Sarcoma, Recurrent Adult Soft Tissue Sarcoma, Locally Advanced Sarcoma, Liposarcoma | Immune Design | November 20, 2015 |
| NCT03448835 | Recruiting | Stomach Cancer, Gastro Esophageal Junction Cancer | The Netherlands Cancer Institute | February 28, 2018 |
| NCT03202316 | Recruiting | Malignant Neoplasm of Breast | M.D. Anderson Cancer Center | June 28, 2017 |
| NCT03465891 | Recruiting | Lymphoma | Memorial Sloan Kettering Cancer Center | March 14, 2018 |
| NCT02807636 | Recruiting | Urothelial Carcinoma | Hoffmann-La Roche | June 21, 2016 |
| NCT03289962 | Recruiting | Melanoma, Non-Small Cell Lung Cancer, Bladder Cancer, Colorectal Cancer, Renal Cancer, Head and Neck Cancer, Other Solid Cancers | Genentech, Inc. | September 21, 2017 |
| NCT03394885 | Not yet recruiting | Ovarian Cancer, Ovarian Neoplasms | Duke University | January 9, 2018 |
| NCT03417544 | Recruiting | HER2-positive Metastatic Breast Cancer, Central Nervous System Metastases | Nancy Lin, MD | January 31, 2018 |
| NCT03138889 | Recruiting | Non-Small Cell Lung Cancer, Urinary Bladder Neoplasms, Neoplasm Metastasis, Melanoma | Nektar Therapeutics | May 3, 2017 |
| NCT02844816 | Recruiting | Recurrent Bladder Urothelial Carcinoma, Stage 0a Bladder Urothelial Carcinoma AJCC v6 and v7, Stage 0is Bladder Urothelial Carcinoma AJCC v6 and v7, Stage I Bladder Cancer with Carcinoma In Situ, Stage I Bladder Urothelial Carcinoma AJCC v6 and v7 | National Cancer Institute (NCI) | July 26, 2016 |
| NCT03102242 | Recruiting | Carcinoma, Non-Small-Cell Lung | Alliance Foundation Trials, LLC. | April 5, 2017 |
| NCT02994576 | Recruiting | Nonsmall Cell Lung Cancer | Gustave Roussy, Cancer Campus, Grand Paris | December 16, 2016 |
| NCT02862275 | Recruiting | Hematopoietic and Lymphoid Cell Neoplasm, Metastatic Malignant Solid Neoplasm, Unresectable Solid Neoplasm | National Cancer Institute (NCI) | August 11, 2016 |
| NCT03133390 | Recruiting | Urothelial Carcinoma | New York University School of Medicine | April 28, 2017 |
| NCT03483012 | Recruiting | Breast Cancer | Dana-Farber Cancer Institute | March 29, 2018 |
| NCT03141684 | Recruiting | Metastatic Alveolar Soft Part Sarcoma | National Cancer Institute (NCI) | May 5, 2017 |
| NCT02458638 | Recruiting | Tumors | Hoffmann-La Roche | June 1, 2015 |
| NCT02891824 | Recruiting | Ovarian Cancer | ARCAGY/ GINECO GROUP | September 8, 2016 |
| NCT03340558 | Not yet recruiting | Metastatic Colorectal Cancer | Niharika Mettu | November 13, 2017 |
| NCT03513952 | Not yet recruiting | Stage III Bladder Cancer AJCC v8, Stage III Renal Pelvis Cancer AJCC v8, Stage III Ureter Cancer AJCC v8, Stage III Urethral Cancer AJCC v8, Stage IV Bladder Cancer AJCC v8, Stage IV Renal Pelvis Cancer AJCC v8, Stage IV Ureter Cancer AJCC v8, Stage IV Urethral Cancer AJCC v8, Stage IVA Bladder Cancer AJCC v8, Stage IVB Bladder Cancer AJCC v8, Urothelial Carcinoma | National Cancer Institute (NCI) | May 2, 2018 |
| NCT02921269 | Active, not recruiting | Cervical Adenocarcinoma, Cervical Adenosquamous Carcinoma, Cervical Squamous Cell Carcinoma, Not Otherwise Specified, Recurrent Cervical Carcinoma, Stage IV Cervical Cancer AJCC v6 and v7, Stage IVA Cervical Cancer AJCC v6 and v7, Stage IVB Cervical Cancer AJCC v6 and v7 | National Cancer Institute (NCI) | October 3, 2016 |
| NCT02992912 | Recruiting | Patients with Metastatic Tumours | Gustave Roussy, Cancer Campus, Grand Paris | December 14, 2016 |
| NCT03267940 | Recruiting | Non-resectable, Intrahepatic, and Extrahepatic Cholangiocarcinoma, Gallbladder Adenocarcinoma | Halozyme Therapeutics | August 31, 2017 |
| NCT02724878 | Recruiting | Advanced Non-Clear Cell Kidney Cancer | Dana-Farber Cancer Institute | March 31, 2016 |
| NCT03024437 | Recruiting | Metastatic Cancer, Renal Cancer | Roberto Pili | January 18, 2017 |
| NCT03422523 | Not yet recruiting | Diffuse Large B Cell Lymphoma, Relapsed Diffuse Large B-Cell Lymphoma, Refractory Diffuse Large B-Cell Lymphoma | University Hospital Southampton NHS Foundation Trust | February 5, 2018 |
| NCT03147040 | Recruiting | Breast Cancer | The Netherlands Cancer Institute | May 10, 2017 |
| NCT02914470 | Active, not recruiting | Breast Cancer, Cervix Cancer, Ovarian Cancer, Endometrial Cancer | The Netherlands Cancer Institute | September 26, 2016 |
| NCT03201458 | Recruiting | Gallbladder Carcinoma, Intrahepatic Cholangiocarcinoma, Non-Resectable Cholangiocarcinoma | National Cancer Institute (NCI) | June 28, 2017 |
| NCT03228537 | Recruiting | Biphasic Mesothelioma, Epithelioid Mesothelioma, Stage I Pleural Malignant Mesothelioma AJCC v7, Stage IA Pleural Malignant Mesothelioma AJCC v7, Stage IB Pleural Malignant Mesothelioma AJCC v7, Stage II Pleural Malignant Mesothelioma AJCC v7, Stage III Pleural Malignant Mesothelioma AJCC v7 | National Cancer Institute (NCI) | July 25, 2017 |
| NCT03206203 | Recruiting | Triple Negative Breast Cancer, Stage IV Breast Cancer, HER2 Negative, Invasive Breast Cancer | Vanderbilt-Ingram Cancer Center | July 2, 2017 |
| NCT03262454 | Not yet recruiting | Small Cell Lung Cancer Recurrent | National Cancer Center, Korea | August 25, 2017 |
| NCT03073525 | Recruiting | Advanced Gynecological Cancers, Ovarian Cancer, Cervical Cancer, Uterine Cancer | Gradalis, Inc. | March 8, 2017 |
| NCT03455556 | Not yet recruiting | Mesothelin Positive, Stage III Non-Small Cell Lung Cancer AJCC v7, Stage IIIA Non-Small Cell Lung Cancer AJCC v7, Stage IIIB Non-Small Cell Lung Cancer AJCC v7, Stage IV Non-Small Cell Lung Cancer AJCC v7 | Mayo Clinic | March 6, 2018 |
| NCT03340376 | Recruiting | Cervical Cancer | Universitaire Ziekenhuizen Leuven | November 13, 2017 |
| NCT03237780 | Recruiting | Metastatic Urothelial Carcinoma, Recurrent Bladder Urothelial Carcinoma, Recurrent Urethral Urothelial Carcinoma, Recurrent Urothelial Carcinoma of the Renal Pelvis and Ureter, Renal Pelvis Urothelial Carcinoma, Stage III Bladder Urothelial Carcinoma AJCC v6 and v7, Stage III Renal Pelvis Cancer AJCC v7, Stage III Ureter Cancer AJCC v7, Stage III Urethral Cancer AJCC v7, Stage IV Bladder Urothelial Carcinoma AJCC v7, Stage IV Renal Pelvis Cancer AJCC v7, Stage IV Ureter Cancer AJCC v7, Stage IV Urethral Cancer AJCC v7, Ureter Urothelial Carcinoma | National Cancer Institute (NCI) | August 3, 2017 |
| NCT03014648 | Recruiting | Advanced Non-small Cell Lung Cancer | University of Pittsburgh | January 9, 2017 |
| NCT03330886 | Not yet recruiting | Urothelial Carcinoma | Hoffmann-La Roche | November 6, 2017 |
| NCT03463057 | Not yet recruiting | NHL, DLBCL | Stichting Hemato-Oncologie voor Volwassenen Nederland | March 13, 2018 |
| NCT02848651 | Active, not recruiting | Non-Small Cell Lung Cancer | Genentech, Inc. | July 28, 2016 |
| NCT03127007 | Recruiting | Rectal Neoplasms | Grand Hôpital de Charleroi | April 25, 2017 |
| NCT02928406 | Recruiting | Urinary Tract Cancer | Hoffmann-La Roche | October 10, 2016 |
| NCT03421288 | Not yet recruiting | Gastric Cancer, Gastroesophageal Junction Adenocarcinoma | IKF Klinische Krebsforschung GmbH at Krankenhaus Nordwest | February 5, 2018 |
| NCT03498222 | Not yet recruiting | Carcinoma, Non-Small-Cell Lung | Queen Mary University of London | April 13, 2018 |
| NCT03438318 | Enrolling by invitation | Non Small Cell Lung Cancer | Checkmate Pharmaceuticals | February 19, 2018 |
| NCT01656642 | Active, not recruiting | Malignant Melanoma | Genentech, Inc. | August 3, 2012 |
| NCT03264066 | Recruiting | Solid Tumors | Hoffmann-La Roche | August 28, 2017 |
| NCT03321695 | Not yet recruiting | Carcinoma, Non-small-cell Lung | Hoffmann-La Roche | October 26, 2017 |
| NCT03519295 | Not yet recruiting | Anal Cancer | GERCOR - Multidisciplinary Oncology Cooperative Group | May 8, 2018 |
| NCT03170960 | Recruiting | Urothelial Carcinoma, Renal Cell Carcinoma, Non-Small Cell Lung Cancer, Castration-resistant Prostate Cancer | Exelixis | May 31, 2017 |
| NCT03208712 | Recruiting | Urothelial Carcinoma | University of Michigan Cancer Center | July 5, 2017 |
| NCT01988896 | Active, not recruiting | Solid Tumors | Hoffmann-La Roche | November 20, 2013 |
| NCT03473756 | Not yet recruiting | Urothelial Carcinoma | Bayer | March 22, 2018 |
| NCT03502785 | Not yet recruiting | Urothelial Carcinoma | Inovio Pharmaceuticals | April 19, 2018 |
| NCT03101280 | Recruiting | Gynecologic Neoplasms | Hoffmann-La Roche | April 5, 2017 |
| NCT03041311 | Active, not recruiting | Small Cell Lung Cancer | G1 Therapeutics, Inc. | February 2, 2017 |
| NCT03434379 | Recruiting | Carcinoma, Hepatocellular | Hoffmann-La Roche | February 15, 2018 |
| NCT03164993 | Recruiting | Cancer, Breast, Triple Negative Breast Cancer | Oslo University Hospital | May 24, 2017 |
| NCT03256344 | Recruiting | Metastatic Triple Negative Breast Cancer, Metastatic Colorectal Cancer | Amgen | August 22, 2017 |
| NCT02926833 | Recruiting | Refractory Diffuse Large B Cell Lymphoma | Kite, A Gilead Company | October 6, 2016 |
| NCT03148418 | Recruiting | Cancer | Hoffmann-La Roche | May 11, 2017 |
| NCT02935361 | Recruiting | Chronic Myelomonocytic Leukemia, Myelodysplastic Syndrome, Recurrent Acute Myeloid Leukemia with Myelodysplasia-Related Changes | University of Southern California | October 17, 2016 |
| NCT02912559 | Recruiting | Colon Adenocarcinoma, DNA Repair Disorder, Lynch Syndrome, Stage III Colon Cancer AJCC v7, Stage IIIA Colon Cancer AJCC v7, Stage IIIB Colon Cancer AJCC v7, Stage IIIC Colon Cancer AJCC v7 | National Cancer Institute (NCI) | September 23, 2016 |
| NCT02708680 | Recruiting | Breast Cancer | Syndax Pharmaceuticals | March 15, 2016 |
| NCT03024216 | Recruiting | Prostate Cancer Metastatic | University of Hawaii | January 18, 2017 |
| NCT02883062 | Recruiting | Estrogen Receptor Negative, HER2/Neu Negative, Invasive Breast Carcinoma, Progesterone Receptor Negative, Stage II Breast Cancer AJCC v6 and v7, Stage IIA Breast Cancer AJCC v6 and v7, Stage IIB Breast Cancer AJCC v6 and v7, Stage III Breast Cancer AJCC v7, Stage IIIA Breast Cancer AJCC v7, Stage IIIB Breast Cancer AJCC v7, Stage IIIC Breast Cancer AJCC v7, Triple-Negative Breast Carcinoma | National Cancer Institute (NCI) | August 30, 2016 |
| NCT03024996 | Recruiting | Renal Cell Carcinoma | Hoffmann-La Roche | January 19, 2017 |
| NCT02031458 | Active, not recruiting | Non-Small Cell Lung Cancer | Hoffmann-La Roche | January 9, 2014 |
| NCT02420821 | Active, not recruiting | Renal Cell Carcinoma | Hoffmann-La Roche | April 20, 2015 |
| NCT02813785 | Active, not recruiting | Carcinoma, Non-Small-Cell Lung | Hoffmann-La Roche | June 27, 2016 |
| NCT03285763 | Recruiting | Carcinoma, Non-Small-Cell Lung | Hoffmann-La Roche | September 18, 2017 |
| NCT03273153 | Recruiting | Advanced BRAFV600 Wild-type Melanoma | Hoffmann-La Roche | September 6, 2017 |
| NCT02655822 | Recruiting | Non-Small Cell Lung Cancer, Malignant Melanoma, Renal Cell Cancer, Triple Negative Breast Cancer, Colorectal Cancer, Bladder Cancer, Metastatic Castration Resistant Prostate Cancer | Corvus Pharmaceuticals, Inc. | January 14, 2016 |
| NCT02927301 | Recruiting | Non-Small Cell Lung Cancer | Genentech, Inc. | October 7, 2016 |
| NCT02471846 | Active, not recruiting | Solid Tumor | Genentech, Inc. | June 15, 2015 |
| NCT03452137 | Recruiting | Locally Advanced Squamous Cell Carcinoma of the Head and Neck (SCCHN) | Hoffmann-La Roche | March 2, 2018 |
| NCT03312530 | Recruiting | Multiple Myeloma | Hoffmann-La Roche | October 17, 2017 |
| NCT03399643 | Recruiting | Urothelial Carcinoma | Hoffmann-La Roche | January 16, 2018 |
| NCT03371017 | Recruiting | Triple Negative Breast Neoplasms | Hoffmann-La Roche | December 13, 2017 |
| NCT03498716 | Not yet recruiting | Triple Negative Breast Cancer | Hoffmann-La Roche | April 17, 2018 |
| NCT03281954 | Recruiting | Triple Negative Breast Cancer | NSABP Foundation Inc | September 13, 2017 |
| NCT02450331 | Recruiting | Carcinoma, Transitional Cell | Hoffmann-La Roche | May 21, 2015 |
| NCT02763579 | Active, not recruiting | Small Cell Lung Carcinoma | Hoffmann-La Roche | May 5, 2016 |
| NCT02323191 | Recruiting | Solid Cancers | Hoffmann-La Roche | December 23, 2014 |
| NCT02715531 | Recruiting | Solid Tumor | Hoffmann-La Roche | March 22, 2016 |
| NCT03125902 | Recruiting | Triple-Negative Breast Cancer | Hoffmann-La Roche | April 24, 2017 |
| NCT02792192 | Recruiting | Bladder Cancer | Hoffmann-La Roche | June 7, 2016 |
| NCT02794571 | Recruiting | Advanced/Metastatic Tumors | Genentech, Inc. | June 9, 2016 |
| NCT02631577 | Active, not recruiting | Lymphoma, Follicular | Hoffmann-La Roche | December 16, 2015 |
| NCT03456063 | Recruiting | Non-Small-Cell Lung | Hoffmann-La Roche | March 7, 2018 |
| NCT02013219 | Active, not recruiting | Non-Small Cell Lung Cancer | Hoffmann-La Roche | December 17, 2013 |
| NCT03292172 | Recruiting | Advanced Ovarian Cancer, Triple Negative Breast Cancer | Hoffmann-La Roche | September 25, 2017 |
| NCT03023423 | Recruiting | Carcinoma, Non-Small-Cell Lung | Janssen Research & Development, LLC | January 18, 2017 |
| NCT03038100 | Recruiting | Fallopian Tube Cancer, Fallopian Tube Cancer, Peritoneal Neoplasms | Hoffmann-La Roche | January 31, 2017 |
| NCT02302807 | Active, not recruiting | Bladder Cancer | Hoffmann-La Roche | November 27, 2014 |
| NCT03197935 | Recruiting | Triple-negative Breast Cancer | Hoffmann-La Roche | June 23, 2017 |
| NCT03357224 | Not yet recruiting | Lymphoma, T-Cell, Cutaneous, Mycosis Fungoides/Sezary Syndrome | European Organisation for Research and Treatment of Cancer - EORTC | November 29, 2017 |
| NCT02410512 | Active, not recruiting | Neoplasms | Genentech, Inc. | April 7, 2015 |
| NCT02659384 | Recruiting | Ovarian Neoplasms | European Organisation for Research and Treatment of Cancer - EORTC | January 20, 2016 |
| NCT02997228 | Recruiting | Colorectal Adenocarcinoma, Mismatch Repair Deficiency, Stage IV Colorectal Cancer AJCC v7, Stage IVA Colorectal Cancer AJCC v7, Stage IVB Colorectal Cancer AJCC v7 | National Cancer Institute (NCI) | December 20, 2016 |
| NCT02605915 | Recruiting | HER2-Positive Metastatic Breast Cancer, HER2-Negative Metastatic Breast Cancer, Locally Advanced or Early Breast Cancer | Hoffmann-La Roche | November 16, 2015 |
| NCT02839707 | Recruiting | Fallopian Tube Clear Cell Adenocarcinoma, Fallopian Tube Endometrioid Adenocarcinoma, High Grade Fallopian Tube Serous Adenocarcinoma, High Grade Ovarian Serous Adenocarcinoma, Ovarian Clear Cell Adenocarcinoma, Ovarian Endometrioid Adenocarcinoma, Ovarian Seromucinous Carcinoma, Primary Peritoneal High Grade Serous Adenocarcinoma, Recurrent Fallopian Tube Carcinoma, Recurrent Ovarian Carcinoma, Recurrent Primary Peritoneal Carcinoma, Undifferentiated Fallopian Tube Carcinoma, Undifferentiated Ovarian Carcinoma | National Cancer Institute (NCI) | July 21, 2016 |
| NCT02304393 | Recruiting | Solid Tumors | Hoffmann-La Roche | December 1, 2014 |
| NCT02431208 | Recruiting | Multiple Myeloma | Hoffmann-La Roche | April 30, 2015 |
| NCT02908672 | Recruiting | Melanoma | Hoffmann-La Roche | September 21, 2016 |
| NCT02425891 | Active, not recruiting | Triple Negative Breast Cancer | Hoffmann-La Roche | April 24, 2015 |
| NCT02814669 | Recruiting | Castrate-Resistant Prostate Cancer | Hoffmann-La Roche | June 28, 2016 |
| NCT02008227 | Active, not recruiting | Non-Squamous Non-Small Cell Lung Cancer | Hoffmann-La Roche | December 11, 2013 |
| NCT03191786 | Recruiting | Non-Small Cell Lung Cancer | Hoffmann-La Roche | June 19, 2017 |
| NCT01898117 | Recruiting | Breast Cancer | The Netherlands Cancer Institute | July 12, 2013 |
| NCT02367781 | Active, not recruiting | Carcinoma, Non-Small-Cell Lung | Hoffmann-La Roche | February 20, 2015 |
| NCT02541604 | Active, not recruiting | Solid Tumor | Hoffmann-La Roche | September 4, 2015 |
| NCT02486718 | Recruiting | Non-Small Cell Lung Cancer | Hoffmann-La Roche | July 1, 2015 |
| NCT02500407 | Recruiting | Lymphocytic Leukemia, Chronic, Lymphoma, Non Hodgkin | Genentech, Inc. | July 16, 2015 |
| NCT02825940 | Recruiting | Neoplasms | Hoffmann-La Roche | July 7, 2016 |
| NCT01984242 | Active, not recruiting | Renal Cell Carcinoma | Hoffmann-La Roche | November 14, 2013 |
| NCT02174172 | Active, not recruiting | Solid Cancers | Hoffmann-La Roche | June 25, 2014 |
| NCT02788279 | Active, not recruiting | Colorectal Cancer | Hoffmann-La Roche | June 2, 2016 |
| NCT03016312 | Recruiting | Prostatic Neoplasms, Castration-Resistant | Hoffmann-La Roche | January 10, 2017 |
| NCT03154827 | Recruiting | Acute Myeloid Leukemia | BioLineRx, Ltd. | May 16, 2017 |
| NCT02657434 | Recruiting | Non-Small Cell Lung Cancer | Hoffmann-La Roche | January 15, 2016 |
| NCT02409342 | Recruiting | Non-Squamous Non-Small Cell Lung Cancer, Squamous Non-Small Cell Lung Cancer | Hoffmann-La Roche | April 6, 2015 |
| NCT02650713 | Active, not recruiting | Advanced/Metastatic Solid Tumors | Hoffmann-La Roche | January 8, 2016 |
| NCT02366143 | Active, not recruiting | Carcinoma, Non-Small-Cell Lung | Hoffmann-La Roche | February 19, 2015 |
| NCT02596971 | Active, not recruiting | Diffuse Large B-Cell Lymphoma, Lymphoma Follicular | Hoffmann-La Roche | November 4, 2015 |
| NCT03178851 | Recruiting | Malignant Melanoma | Hoffmann-La Roche | June 7, 2017 |
| NCT02367794 | Active, not recruiting | Squamous Non-Small Cell Lung Cancer | Hoffmann-La Roche | February 20, 2015 |
| NCT02220842 | Active, not recruiting | Lymphoma | Hoffmann-La Roche | August 20, 2014 |
| NCT02350673 | Recruiting | Solid Tumors | Hoffmann-La Roche | January 30, 2015 |
| NCT02322814 | Recruiting | Breast Cancer | Hoffmann-La Roche | December 23, 2014 |
| NCT01375842 | Active, not recruiting | Tumors, Hematologic Malignancies | Genentech, Inc. | June 17, 2011 |
| NCT02873195 | Recruiting | Recurrent Colorectal Carcinoma, Stage IV Colorectal Cancer AJCC v7, Stage IVA Colorectal Cancer AJCC v7, Stage IVB Colorectal Cancer AJCC v7 | Academic and Community Cancer Research United | August 19, 2016 |
| NCT02924883 | Active, not recruiting | Metastatic Breast Cancer | Hoffmann-La Roche | October 5, 2016 |
| NCT02729896 | Active, not recruiting | Lymphoma | Hoffmann-La Roche | April 6, 2016 |
| NCT03371992 | Enrolling by invitation | Non Small Cell Lung Cancer | Nilogen Oncosystems | December 13, 2017 |
| NCT03232593 | Recruiting | Bladder Cancer, Non-Small Cell Lung Cancer | Hoffmann-La Roche | July 28, 2017 |
| NCT02478099 | Active, not recruiting | Bladder Cancer, Non Small Cell Lung Cancer, Breast Cancer | University Medical Center Groningen | June 23, 2015 |
| NCT01903993 | Active, not recruiting | Non-Small Cell Lung Cancer | Hoffmann-La Roche | July 19, 2013 |
| NCT02091141 | Recruiting | Neoplasms | Genentech, Inc. | March 19, 2014 |
| NCT02525757 | Active, not recruiting | Lung Cancer, Non-Small Cell Lung Cancer | M.D. Anderson Cancer Center | August 17, 2015 |
| NCT02453984 | Recruiting | Breast Cancer, Bladder Cancer, Non Small Cell Lung Cancer | University Medical Center Groningen | May 27, 2015 |
| NCT02530489 | Recruiting | Breast Cancer | M.D. Anderson Cancer Center | August 21, 2015 |
| NCT02451423 | Recruiting | Carcinoma, Transitional Cell | University of California, San Francisco | May 22, 2015 |
| NCT02400814 | Recruiting | Recurrent Non-Small Cell Lung Carcinoma, Stage IV Non-Small Cell Lung Cancer | University of California, Davis | March 27, 2015 |
| NCT02662309 | Recruiting | Bladder Cancer | Queen Mary University of London | January 25, 2016 |
| NCT02716038 | Recruiting | Carcinoma, Non-Small-Cell Lung | Columbia University | March 22, 2016 |
| NCT02463994 | Active, not recruiting | Non-small Cell Lung Cancer | University of Michigan Cancer Center | June 8, 2015 |
| NCT02630186 | Active, not recruiting | Non-small Cell Lung Cancer | Clovis Oncology, Inc. | December 15, 2015 |
| NCT02777710 | Recruiting | Pancreatic Cancer, Pancreatic Cancer, Metastatic Cancer, Advanced Cancer | Centre Leon Berard | May 19, 2016 |
| NCT03063762 | Recruiting | Renal Cell Carcinoma | Hoffmann-La Roche | February 24, 2017 |
| NCT03179943 | Recruiting | Urothelial Carcinoma | Fox Chase Cancer Center | June 7, 2017 |
| NCT02599454 | Recruiting | Stage I Non-Small Cell Lung Cancer | University of California, Davis | November 6, 2015 |
| NCT02876224 | Recruiting | Colorectal Cancer | Hoffmann-La Roche | August 23, 2016 |
| NCT03311334 | Recruiting | Neoplasms, Melanoma, Non Small Cell Lung Cancer, Head and Neck Squamous Cell Carcinoma, Renal Cell Carcinoma, Urothelial Neoplasm | Boston Biomedical, Inc | October 17, 2017 |
| NCT01633970 | Active, not recruiting | Cancer | Genentech, Inc. | July 6, 2012 |
| NCT03050060 | Recruiting | Metastatic Renal Cell Cancer, Recurrent Melanoma, Recurrent Non-Small Cell Lung Carcinoma, Recurrent Renal Cell Carcinoma, Stage IV Cutaneous Melanoma AJCC v6 and v7, Stage IV Non-Small Cell Lung Cancer AJCC v7, Stage IV Renal Cell Cancer AJCC v7 | University of Washington | February 10, 2017 |
| NCT03087864 | Recruiting | Esophageal Cancer Stage II, Esophageal Cancer Stage III | Academisch Medisch Centrum - Universiteit van Amsterdam (AMC-UvA) | March 23, 2017 |
| NCT03363867 | Not yet recruiting | Ovarian Cancer, Fallopian Tube Cancer, Primary Peritoneal Carcinoma | Peter MacCallum Cancer Centre, Australia | December 6, 2017 |
| NCT03316417 | Not yet recruiting | Adverse Effect, Renal Toxicity | Hospices Civils de Lyon | October 20, 2017 |
| NCT03059667 | Active, not recruiting | Small Cell Lung Cancer, Small Cell Lung Cancer Limited Stage, Small Cell Lung Cancer Extensive Stage | Intergroupe Francophone de Cancerologie Thoracique | February 23, 2017 |
| NCT03464942 | Not yet recruiting | Breast Cancer | Peter MacCallum Cancer Centre, Australia | March 14, 2018 |
| NCT02902029 | Recruiting | Malignant Melanoma | University Hospital, Essen | September 15, 2016 |
| NCT03386721 | Recruiting | Solid Tumors | Hoffmann-La Roche | December 29, 2017 |
| NCT03430518 | Not yet recruiting | HER2-Negative Metastatic Breast Cancer, Recurrent Ovarian Cancer | Amy Tiersten | February 13, 2018 |
| NCT03046953 | Recruiting | T-Cell Lymphoma Relapsed, T-Cell Lymphoma Refractory | University of Birmingham | February 8, 2017 |
| NCT02871323 | Active, not recruiting | Polycythemia Vera, Post-Polycythemic Myelofibrosis Phase, Primary Myelofibrosis | Northwestern University | August 18, 2016 |
| NCT02811497 | Recruiting | Microsatellite Stable Colorectal Carcinoma, Platinum Resistant Epithelial Ovarian Cancer Type II, Estrogen Receptor Positive and HER2 Negative Breast Cancer | University Health Network, Toronto | June 23, 2016 |
| NCT02886065 | Active, not recruiting | Smoldering Multiple Myeloma | Massachusetts General Hospital | September 1, 2016 |
| NCT03271372 | Recruiting | Stage III Merkel Cell Carcinoma AJCC v7, Stage IIIB Merkel Cell Carcinoma AJCC v7 | University of Washington | September 5, 2017 |
| NCT02733042 | Active, not recruiting | Lymphoma, Leukemia, Lymphocytic, Chronic, B-Cell | Celgene | April 11, 2016 |
| NCT03390296 | Recruiting | Acute Myeloid Leukemia | M.D. Anderson Cancer Center | January 4, 2018 |
| NCT03212469 | Recruiting | Head and Neck Squamous Cell Carcinoma, Lung Cancer, Oesophageal Cancer | Gustave Roussy, Cancer Campus, Grand Paris | July 11, 2017 |
| NCT03395899 | Recruiting | Breast Cancer, Estrogen Receptor-positive Breast Cancer | Queen Mary University of London | January 10, 2018 |
| NCT02311361 | Recruiting | Pancreatic Neoplasms, Pancreatic Cancer, Cancer of Pancreas, Cancer of the Pancreas, Pancreas Cancer | National Cancer Institute (NCI) | December 8, 2014 |
| NCT03050814 | Recruiting | Colorectal Cancer | National Cancer Institute (NCI) | February 13, 2017 |
| NCT03288545 | Recruiting | Carcinoma, Transitional Cell, Urinary Bladder Neoplasms, Urologic Neoplasms, Renal Pelvis Neoplasms, Urothelial Cancer, Ureteral Neoplasms, Urethral Neoplasms | Astellas Pharma Global Development, Inc. | September 20, 2017 |
| NCT03399071 | Recruiting | Gastric Adenocarcinoma, Oesophageal Adenocarcinoma | Royal Marsden NHS Foundation Trust | January 16, 2018 |
| NCT02314481 | Recruiting | Non-small Cell Lung Cancer | University College, London | December 11, 2014 |
Approved Drugs of Atezolizumab**
| INN (trade name) | Therapeutic area | Dose | Strength | Route | Company | Marketing start | Market |
| Tecentriq | Locally Advanced or Metastatic Urothelial Carcinoma; Metastatic Non-Small Cell Lung Cancer | Injection, Solution | 60 mg/mL | Intravenous | Genentech, Inc. | May 18, 2016 |

|
| Tecentriq | Locally Advanced or Metastatic Urothelial Carcinoma | Injection, Solution | 60 mg/mL | Intravenous | Hoffmann La Roche Limited | May 2, 2017 |

|
| Tecentriq | Locally Advanced or Metastatic Non-Small Cell Lung Cancer | Injection, Solution | 60 mg/mL | Intravenous | Roche Products Pty Ltd | July 27, 2017 |

|
| Tecentriq | Carcinoma, Non-Small-Cell Lung; Carcinoma, Transitional Cell | Injection, Solution | 60 mg/mL | Intravenous | Roche Registration GmbH | September 21, 2018 |

|
Resources
* The table was excerpted from the following website
https://clinicaltrials.gov/ct2/results?cond=&term=Atezolizumab
** Information presented in the table were collected from the following websites:
https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=761034
https://health-products.canada.ca/dpd-bdpp/info.do?lang=en&code=95089
http://search.tga.gov.au/s/search.html?collection=tga-artg&profile=record&meta_i=277120
http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/004143/human_med_002166.jsp
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.



