Brodalumab Overview
Introduction of Brodalumab
Brodalumab is a fully human anti-interleukin 17 receptor A (IL-17RA) IgG2 monoclonal antibody. It been investigated in a range of disease including psoriasis, psoriatic arthritis, rheumatoid arthritis, inflammatory bowel disease and asthma. In 2016, brodalumab was approved for marketing in Japan for the treatment of psoriatic arthritis, psoriatic erythroderma, pustular psoriasis, and psoriasis vulgaris. In 2017, it was successively approved by U.S. Food and Drug Administration and European Medicines Agency for the treatment of moderate to severe plaque psoriasis.
Mechanism of Action of Brodalumab
Psoriasis is a complex disease in which the T helper (Th) 17 cells appears to be crucial for its pathogenic mechanisms. Interleukin 17 (IL-17) produced by Th17 cells plays a crucial role in the inflammatory process by stimulating production of several proinflammatory cytokines and chemokines by keratinocytes, dendritic cells, and other immune cells. IL-17 subtypes (IL-17A and IL-17F) interact with a group of transmembrane receptors including IL-17RA. By binding to IL-17RA, Brodalumab inhibits its binding to the IL-17A, thereby inhibiting its pro-inflammatory downstream effects on keratinocytes.
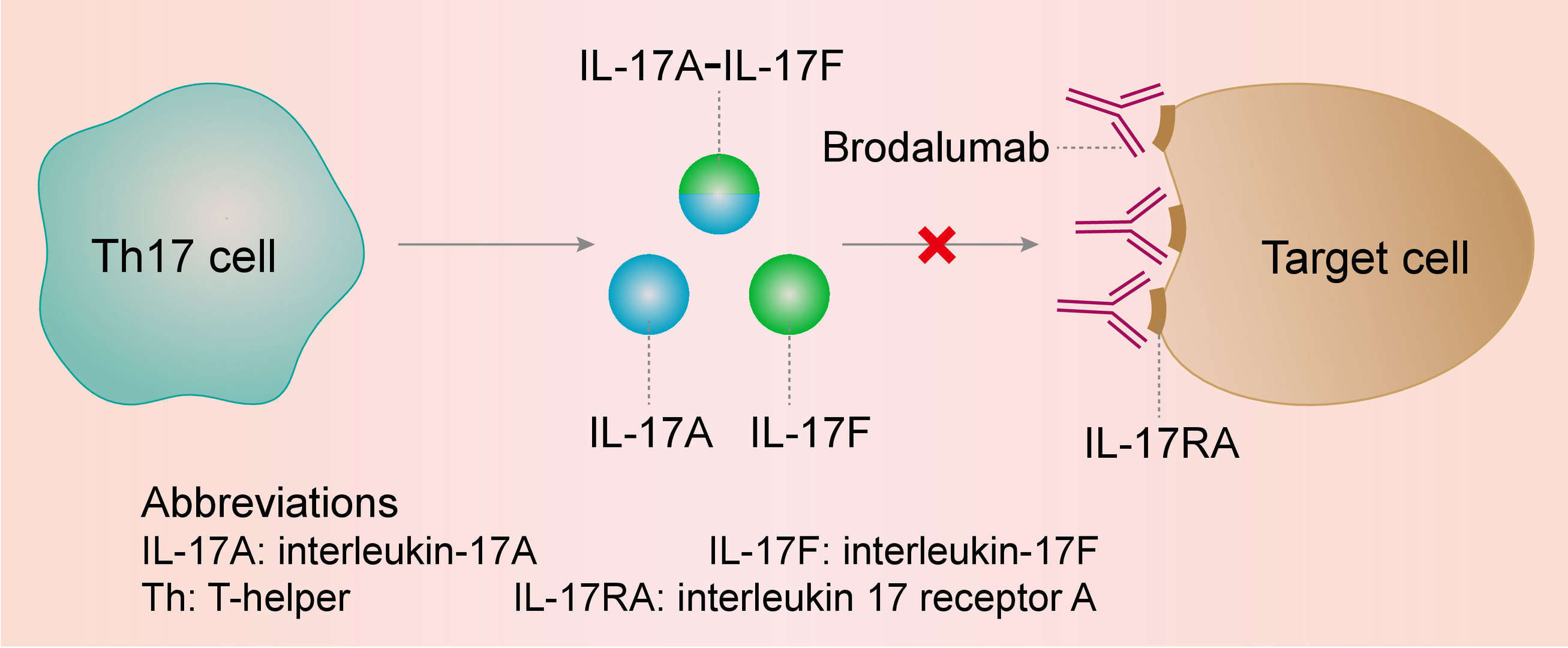 Fig.1 Mechanism of action of Brodalumab
Fig.1 Mechanism of action of Brodalumab
Clinical Projects of Brodalumab*
| NCT ID | Status | Conditions | Lead Sponsor | Update Time |
| NCT03403036 | Recruiting | Psoriasis | Icahn School of Medicine at Mount Sinai | January 18, 2018 |
| NCT03478280 | Not yet recruiting | Psoriasis | Aarhus University Hospital | March 27, 2018 |
| NCT03331835 | Recruiting | Psoriasis Vulgaris | LEO Pharma | November 6, 2017 |
| NCT03254667 | Not yet recruiting | Psoriasis | Valeant Pharmaceuticals International, Inc. | August 18, 2017 |
| NCT03240809 | Not yet recruiting | Psoriasis | Valeant Pharmaceuticals International, Inc. | August 7, 2017 |
| NCT02982005 | Active, not recruiting | Moderate to Severe Plaque Psoriasis | Kyowa Hakko Kirin Korea Co., Ltd. | December 5, 2016 |
| NCT02985983 | Recruiting | Axial Spondyloarthritis | Kyowa Hakko Kirin Co., Ltd | December 7, 2016 |
Approved Drugs of Brodalumab**
| INN (trade name) | Therapeutic area | Dose | Strength | Route | Company | Marketing start | Market |
| Lumicef | Plaque psoriasis, Psoriatic arthritis, Pustular Psoriasis, Erythrodermic psoriasis | Injection, Solution | 140 mg/mL | Subcutaneous | Kyowa Hakko Kirin Co., Ltd. | July 04, 2016 |

|
| Siliq | Moderate to severe plaque psoriasis | Injection, Solution | 140 mg/mL | Subcutaneous | Valeant Luxembourg | February 15, 2017 |

|
| Kyntheum | Moderate to severe plaque psoriasis | Injection, Solution | 140 mg/mL | Subcutaneous | LEO Pharma A/S | July 17, 2017 |

|
Resources
* The table was excerpted from the following website
https://clinicaltrials.gov/ct2/results?cond=&term=Brodalumab
** Information presented in the table were collected from the following websites:
http://www.pmda.go.jp/PmdaSearch/iyakuDetail/GeneralList/3999441G1
https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=761032
http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/003959/human_med_002054.jsp
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.



