Crenezumab Overview
Introduction of Crenezumab
Crenezumab is a fully humanized monoclonal antibody (from mouse) against human 1-42 β-amyloid (Aβ), which is being investigated as a treatment of Alzheimer's disease. Crenezumab recognizes multiple forms of aggregated Aβ, including oligomeric and fibrillar species and amyloid plaques with high affinity, and monomeric Aβ with low affinity. Crenezumab remains the only antibody that targets the mid-region of Aβ peptide and binds to multiple aggregated forms with dissociating effects.
Mechanism of Action of Crenezumab
The precise mechanism by which Crenezumab exerts its therapeutic effects in AD is unknown. Crenezumab is highly homologous to solanezumab, another monoclonal antibody targeting Aβ peptides. It was engineered to clear excess Aβ while exerting reduced subsequent effector function on microglia; the rationale is to stimulate amyloid phagocytosis while limiting release of inflammatory cytokines as a way to avoid side effects such as vasogenic edema.
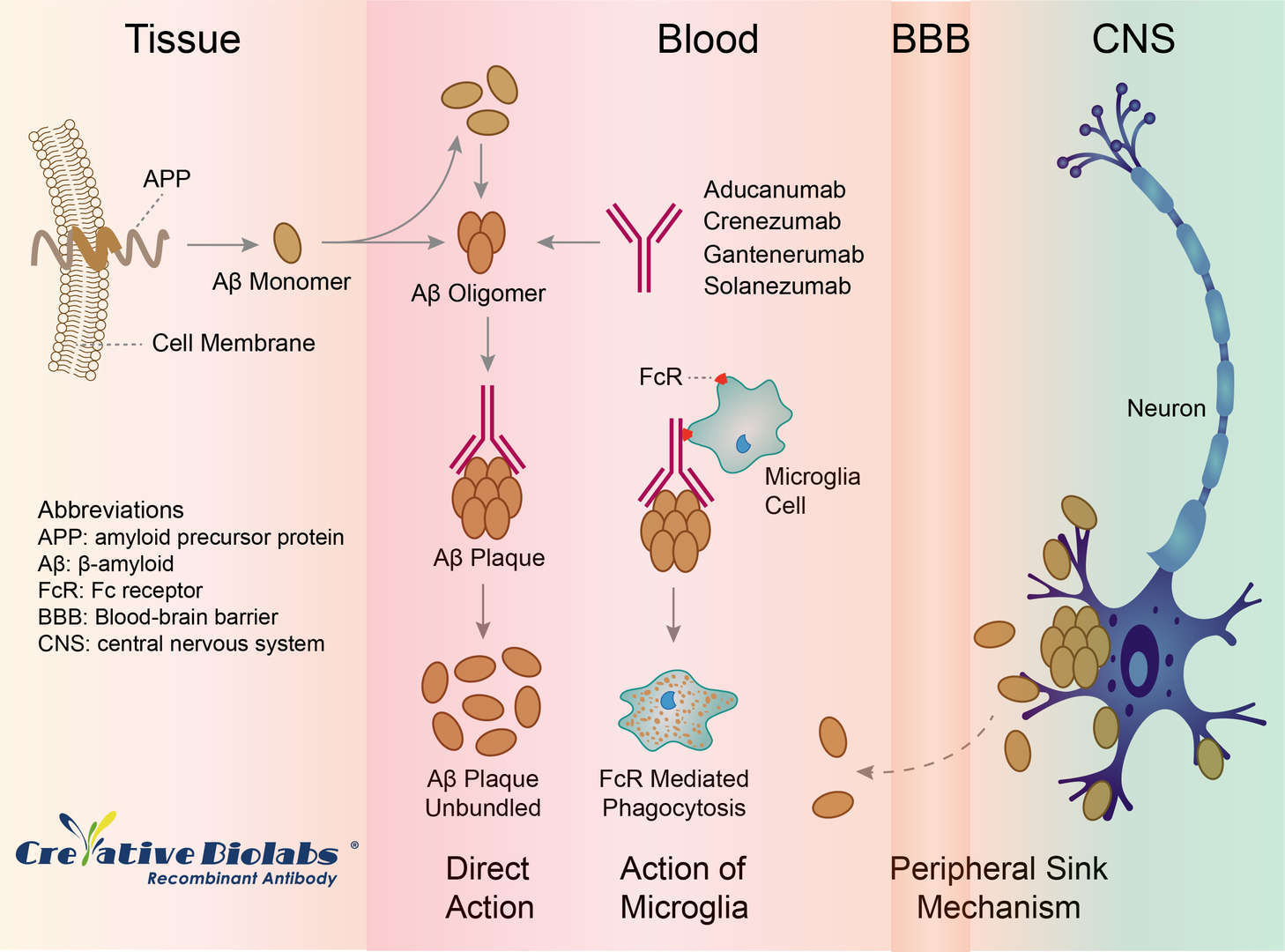 Fig.1 Mechanism of Action of Crenezumab
Fig.1 Mechanism of Action of Crenezumab
Clinical Projects of Crenezumab*
| NCT ID | Status | Conditions | Lead Sponsor | Update Time |
| NCT03491150 | Recruiting | Alzheimer's Disease | Hoffmann-La Roche | April 9, 2018 |
| NCT03114657 | Recruiting | Alzheimer's Disease | Hoffmann-La Roche | April 14, 2017 |
| NCT02353598 | Active, not recruiting | Alzheimer's Disease | Genentech, Inc. | February 3, 2015 |
| NCT02670083 | Active, not recruiting | Alzheimer's Disease | Hoffmann-La Roche | February 1, 2016 |
| NCT01998841 | Active, not recruiting | Alzheimer's Disease | Genentech, Inc. | December 2, 2013 |
Resource
* The table was excerpted from the following website
https://clinicaltrials.gov/ct2/results?cond=&term=Crenezumab
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.



