Eculizumab Overview
Introduction of Eculizumab
Eculizumab is a recombinant humanized monoclonal antibody produced by murine myeloma cell culture and purified by standard bioprocess technology. Eculizumab contains human constant regions from human IgG2 sequences and human IgG4 sequences and murine complementarity-determining regions grafted onto the human framework light- and heavy-chain variable regions. Eculizumab is composed of two 448 amino acid heavy chains and two 214 amino acid light chains and has a molecular weight of approximately 148 kDa. This drug is a monoclonal antibody directed against the complement protein C5. This antibody blocks the cleavage of C5 and halts the process of complement-mediated cell destruction. Eculizumab has been shown to be effective in treating paroxysmal nocturnal hemoglobinuria. In March 2007, eculizumab was approved by the FDA as a treatment for adult patients with generalized myasthenia gravis (gMG) who are anti-acetylcholine receptor (AchR) antibody-positive. In the Phase 3 REGAIN study and its ongoing open-label extension study, it demonstrated treatment benefits for patients with anti-AchR antibody-positive gMG.
Mechanism of Action of Eculizumab
Myasthenia gravis (MG) is a debilitating, chronic and progressive autoimmune neuromuscular disease that can occur at any age. It typically begins with weakness in the muscles that control the movements of the eyes and eyelids, and often progresses to the more severe and generalized form, known as gMG, with weakness of the head, neck, trunk, limb and respiratory muscles. A genetic mutation in paroxysmal nocturnal hemoglobinuria (PNH) patients leads to the generation of populations of abnormal RBCs (known as PNH cells) that are deficient in terminal complement inhibitors (CD-59), rendering PNH RBCs sensitive to persistent terminal complement-mediated destruction. The destruction and loss of these PNH cells (intravascular hemolysis) results in low RBC counts (anemia) and also fatigue, difficulty in functioning, pain, dark urine, shortness of breath, and blood clots. Soliris® is a complement inhibitor that works by inhibiting the terminal part of the complement cascade, a part of the immune system that, when activated in an uncontrolled manner, plays a role in serious ultra-rare disorders like paroxysmal nocturnal hemoglobinuria (PNH), atypical hemolytic uremic syndrome (aHUS) and anti-acetylcholine receptor (AchR) antibody-positive myasthenia gravis (MG). Eculizumab, the active ingredient in Soliris, is a monoclonal antibody that binds to the complement protein C5 specifically and with high affinity, thereby inhibiting its cleavage to C5a and C5b and subsequent generation of the terminal complement complex C5b-9. This interference prevents the destruction of red blood cells (hemolysis) and therefore results in stabilization of hemoglobin and a decrease in the need for blood transfusions in persons with paroxysmal nocturnal hemoglobinuria (PNH). Soliris inhibits terminal complement mediated intravascular hemolysis in PNH patients and therefore the destruction of PNH erythrocytes that lack complement protection with CD-59.
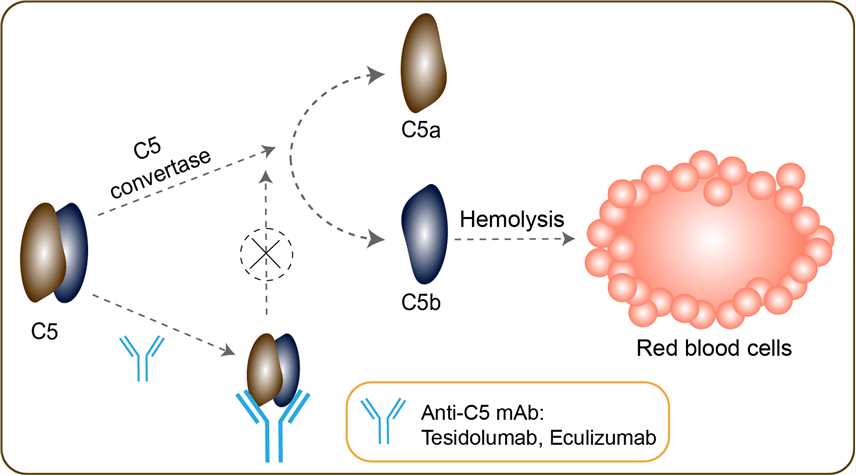
Fig.1 Mechanism of action of Eculizumab
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

