Emibetuzumab Overview
Introduction of Emibetuzumab
Emibetuzumab (as known as LY2875358) is a humanized immunoglobulin G4 monoclonal bivalent anti-MET antibody that blocks MET signaling. This drug has been used in trials studying the treatment of Advanced Cancer, Renal Cell Carcinoma, Hepatocellular Cancer, Gastric Adenocarcinoma, and Non-Small Cell Lung Cancer, among others. In preclinical models, emibetuzumab inhibited growth of tumors that are dependent on MET, including tumors that exhibit HGF-dependent and HGF-independent biology. Administration of a single dose of emibetuzumab resulted in continuous reduction of MET protein expression for 14 days in mouse xenografts. Emibetuzumab demonstrated dose-dependent, single-agent activity in various MET-expressing tumor models, and additive effects were observed in combination with erlotinib for reducing tumor volume in lung cancer mouse xenograft models. In Phase 1 dose escalation studies, emibetuzumab monotherapy was well tolerated in patients with advanced solid tumors, including gastric cancer, with no dose-limiting toxicities.
Mechanism of Action of Emibetuzumab
The mesenchymal–epithelial transition factor (MET) receptor is a tyrosine kinase receptor and its only known ligand is hepatocyte growth factor (HGF). HGF/MET signaling is involved in embryogenesis and responses to tissue damage, and MET is broadly expressed in normal adult tissues. Activation of MET signaling can occur via a ligand-dependent mechanism, by HGF, or ligand-independent mechanisms, including overexpression of the MET protein, amplification of the MET gene (c-met), or c-met mutations. MET is involved in many mechanisms of cancer proliferation and metastasis. Inappropriate activation of MET can be induced by HGF-independent mechanisms such as gene amplification, specific genetic mutations, and transcriptional up-regulation, or by HGF-dependent autocrine or paracrine mechanisms. Aberrant MET signaling has been described to be involved in tumor growth and invasion, angiogenesis, metastasis, and resistance to therapy. MET expression has been reported in many tumor types, including gastric cancer. It has been suggested that c-met amplification and overexpression of MET protein in gastric cancer correlate with poor patient outcomes. Clinical studies with MET-targeting agents have demonstrated a role for MET as a predictive biomarker in patients with gastric cancer. Emibetuzumab is a humanized IgG4 bivalent monoclonal antibody that inhibits HGF-dependent and HGF-independent MET signaling via two distinct mechanisms: it binds to the extracellular domain of MET, preventing HGF from binding to MET and thereby inhibiting ligand-dependent activation of MET. Moreover, emibetuzumab triggers MET receptor internalization and downregulation of total membrane MET expression, leading to inhibition of ligand-independent activation of MET signaling, as well. In HGF-dependent MET activation, emibetuzumab blocks HGF binding to MET, HGF-induced MET phosphorylation and tumor growth both in cell culture and in mouse xenograft models, resembling activities of a humanized one-armed 5D5 MET antibody (monovalent antibody similar to Onartuzumab). In contrast to other bivalent MET antibodies, emibetuzumab has no or otherwise negligible agonist activity and does not stimulate biological activities such as cell proliferation, scattering, invasion, tubulogenesis, apoptosis protection or angiogenesis in various HGF responsive cells. These findings indicate that emibetuzumab has a different mechanism of action from the humanized one-armed 5D5 MET antibody. Emibetuzumab may be a promising therapy for treatment of patients whose tumors are driven by HGF-dependent and HGF-independent MET activation.
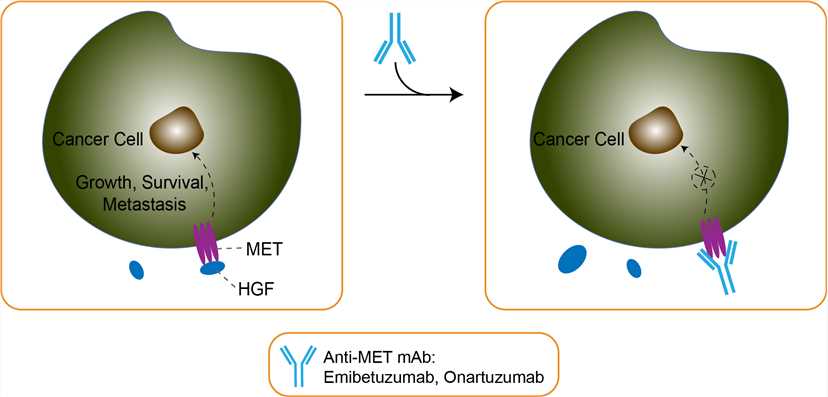
Fig 1. Mechanism of Action of Emibetuzumab
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

