Galcanezumab Overview
Introduction of Galcanezumab
Galcanezumab (LY2951742) is a humanized monoclonal antibody (mAb) directed against calcitonin gene-related polypeptides (CGRPs) α and β, which are pivotal in the pathophysiology of migraine headaches and represent a promising target for migraine treatment. As of July 2017, three Phase III clinical trials have been completed.
Mechanism of Action of Galcanezumab
Migraine headache is associated with activation of trigeminal durovascular nociceptive afferents and release of CGRP. CGRP is a 37-amino acid neuropeptide that exists in two isoforms that differ by three amino acids in the human and have different tissue distributions. Galcanezumab binds to both α and β isoforms of CGRP and inhibits the interaction between CGRP and its’ receptor, thereby shows efficacy in the treatment of migraine. In the peripheral nervous system, α-CGRP is predominantly expressed in the nociceptive Ad and C fibers, with widespread innervation throughout the body, including an extensive perivascular distribution; β-CGRP is the predominant form expressed in the enteric nervous system. The distribution of CGRP and the CGRP receptor in the central nervous system is exquisitely complex and still largely enigmatic. CGRP is also expressed in nonneuronal tissues of which less is known. CGRP exerts its action on the CGRP receptor, also called the canonical CGRP receptor. The CGRP receptor is a heterodimer of calcitonin receptor-like receptor (CLR) and receptor activity modifying protein 1 (RAMP1). In addition, the receptor component protein needs to be present to form a functional CGRP receptor. CGRP also has a high potency at the amylin 1 receptor, a heterodimer of CLR and RAMP2, and may activate it physiologically. Established biological roles for CGRP are vasodilator activity and (modulation of) nociception. CGRP is the most potent microvascular dilator currently known, and its vasodilator activity is observed in the cerebral, coronary and kidney vascular beds. Additional roles of CGRP in biology and pathophysiology have been discussed, including in hypertension, wound healing and diabetes.
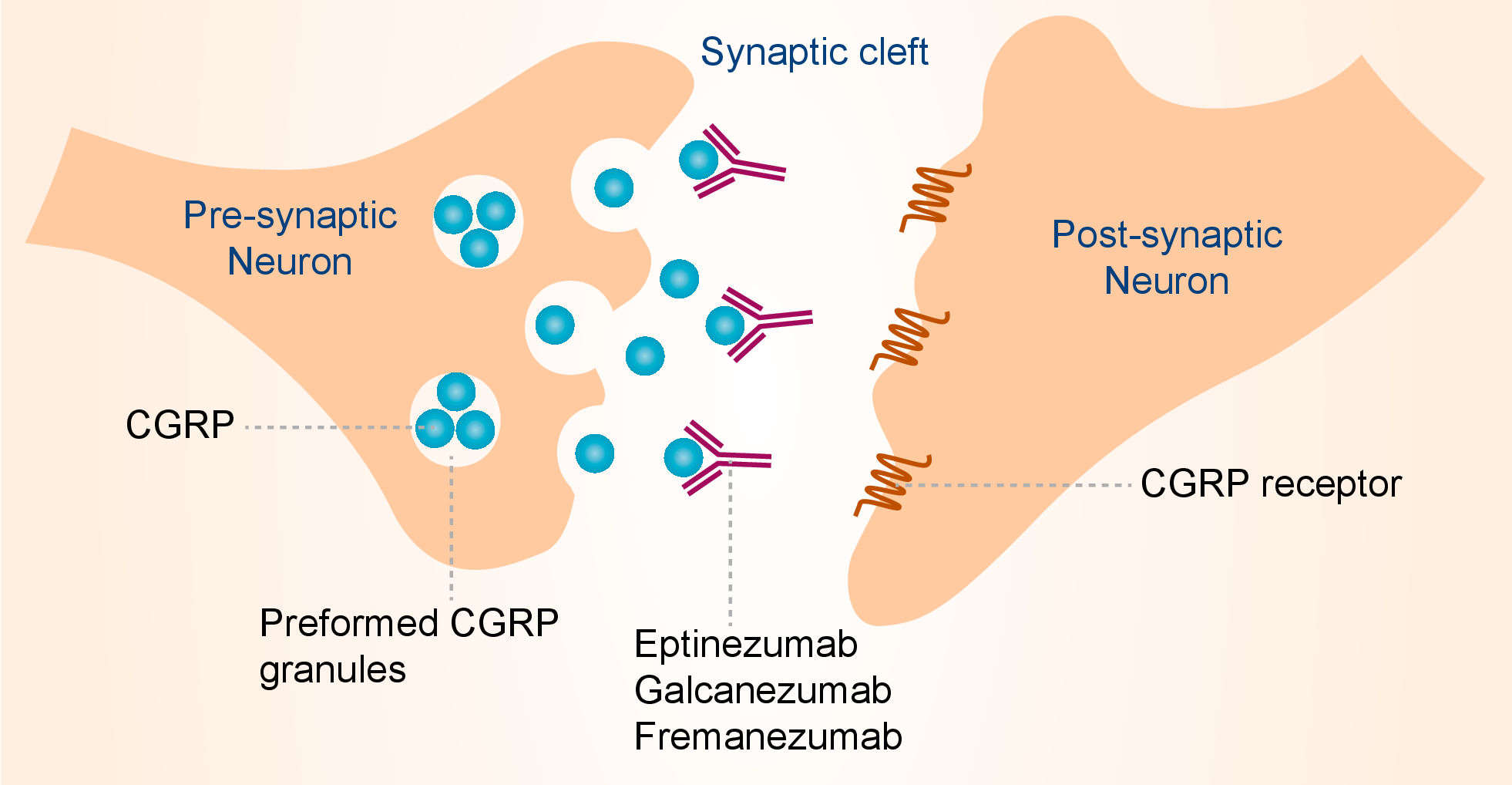 Fig.1 Mechanism of action of galcanezumab
Fig.1 Mechanism of action of galcanezumab
Clinical Projects of Galcanezumab*
| NCT ID | Status | Conditions | Lead Sponsor | Update Time |
| NCT03559257 | Not yet recruiting | Migraine | Eli Lilly and Company | June 18, 2018 |
| NCT02959190 | Active, not recruiting | Migraine | Eli Lilly and Company | November 8, 2016 |
| NCT03432286 | Recruiting | Episodic Migraine | Eli Lilly and Company | February 14, 2018 |
| NCT02959177 | Active, not recruiting | Migraine | Eli Lilly and Company | November 8, 2016 |
| NCT02797951 | Enrolling by invitation | Episodic Cluster Headache | Eli Lilly and Company | June 14, 2016 |
| NCT02614261 | Active, not recruiting | Chronic Migraine | Eli Lilly and Company | November 25, 2015 |
| NCT02614196 | Active, not recruiting | Migraine | Eli Lilly and Company | November 25, 2015 |
| NCT02614287 | Active, not recruiting | Migraine | Eli Lilly and Company | November 25, 2015 |
* The table was excerpted from the following website
https://clinicaltrials.gov/ct2/results?cond=&term=Galcanezumab
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

