Gemtuzumab Ozogamicin Overview
Introduction of Gemtuzumab Ozogamicin
Gemtuzumab ozogamicin is a humanized IgG4 anti-CD33 monoclonal antibody (hP67.6) conjugated to N-acetyl-γ calicheamicin dimethyl hydrazide (NAc-gamma calicheamicin DMH), a derivative of calicheamicin. The constant region and framework regions of the hP67.6 antibody contain human sequences, whereas the complementarity-determining regions (CDRs) are derived from a murine antibody (p67.6) that binds CD33. Calicheamicin is a naturally-occurring hydrophobic enediyne antibiotic that was isolated from the actinomycete Micromonospora echinospora calichensis. Conjugation with the antibody is obtained by covalent linkage (condensation) of a bifunctional linker, 4-(4-acetylphenoxy)butanoic acid (AcBut linker), which allows the most favourable balance between hydrolytic stability in physiological buffers (pH 7.4) and efficient drug release at the pH of lysosomes ( ∼ 4). In the United States (US), Gemtuzumab ozogamicin was approved under an accelerated-approval process by the Food and Drug Administration (FDA) in 2000 for use in patients over the age of 60 with relapsed acute myelogenous leukemia (AML); or those who are not considered candidates for standard chemotherapy. The accelerated approval was based on the surrogate endpoint of response rate. It was the first antibody-drug conjugate to be approved.
Mechanism of Action of Gemtuzumab Ozogamicin
The CD33 antigen is a sialic acid-dependent adhesion protein that is specific for myeloid cells. CD33 is expressed in approximately 90% of AML cases, as defined by the presence of the antigen on > 20% of the leukemic blasts but not on normal CD34+ pluripotent hematopoietic stem cells or non-hematopoietic tissues. Gemtuzumab ozogamicin consists of a humanized anti-CD33 antibody, produced from a mammalian myeloma cell line, covalently linked to a semisynthetic derivative of a potent cytotoxic enediyne antibiotic called calicheamicin. Gemtuzumab ozogamicin targets CD33-expressing cells. Binding to the antigen is followed by endocytosis. The covalent link is cleaved inside lysosomes, allowing calicheamicin release. Calicheamicin binds to DNA within the minor groove and causes double-strand breaks and ultimately, cell apoptosis.
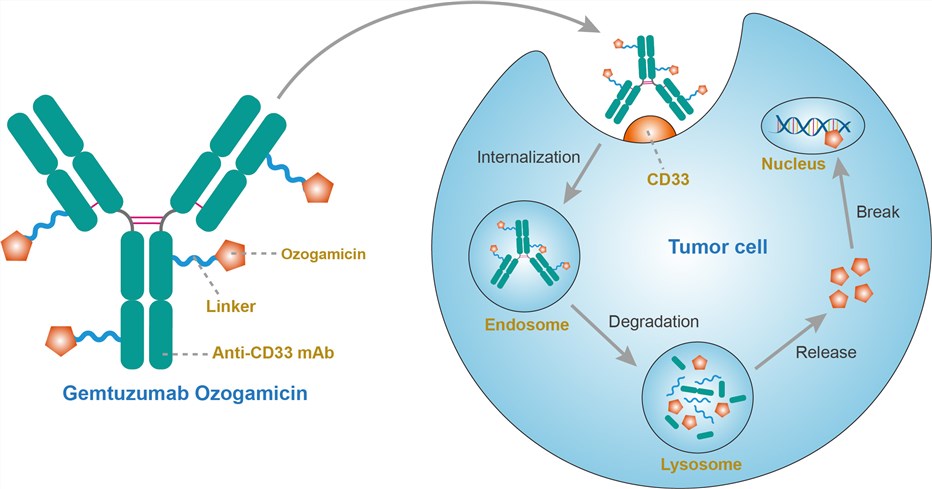 Fig.1 Mechanism of action of gemtuzumab ozogamicin
Fig.1 Mechanism of action of gemtuzumab ozogamicin
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.



