Gevokizumab Overview
Introduction of Gevokizumab
Gevokizumab (Box 1) is a potent monoclonal antibody (mAb) with unique allosteric modulating properties and the potential to treat patients with a wide variety of diseases. Gevokizumab binds strongly to interleukin-1 beta (IL-1β), a pro-inflammatory cytokine, which has been implicated in cardiovascular conditions, lung cancer, and auto-inflammatory diseases. Gevokizumab has been used in trials studying the treatment of acne vulgaris, osteoarthritis, Bechet’s uveitis, pyoderma gangrenosum, and Bechet’s disease uveitis, among others.
Mechanism of Action of Gevokizumab
Gevokizumab can neutralize human IL-1β by binding to it. IL-1 is a proinflammatory cytokine that plays a role in the immune response to microorganisms, trauma and stress, while this cytokine is additionally involved in tissue destruction and fibrosis. There are two distinct IL-1 genes encoding the production of IL-1α and IL-1β, namely gene IL1A and IL1B. IL-1 receptor type 1 (IL-1R1) is a virtually ubiquitous cell-surface receptor. Each IL-1 binds to this receptor. After binding, IL-1 triggers a cascade of inflammatory mediators, chemokines and other cytokines. Gevokizumab competes with IL-1R1 to bind to IL-1β and inhibits the IL-1β/IL-1R1 induced downstream pathways. In terms of novel mechanism, the action of gevokizumab does not involve simple competitive receptor binding but allows a context-dependent attenuation of ligand activity. This is ascribed to the fact that the epitope of gevokizumab is proximal to, but does not overlap with, the receptor interface, without specifically inhibiting the region required for receptor binding. Indeed, two of the residues of IL-1b that are important for XOMA 052 binding (E96 and K97) are located in the G loop of the mature IL-1b protein, which is adjacent to the region that contacts the receptor, and a part of this loop is important for receptor binding. The third residue important for XOMA 052 binding (Q116) is located near this loop, and gevokizumab is the first reported example of a therapeutic antibody binding to this region. Finally, the magnitude of the diminution in signaling as accomplished by gevokizumab is independent of antibody concentration, provided all available IL-1b are bound. Under this condition, signaling output depends on the concentrations of ligand and the other components of the signaling system (e.g., IL-1Rα, IL-1 receptors, etc.). As a result, very low receptor occupancy can achieve efficacious signaling transduction.
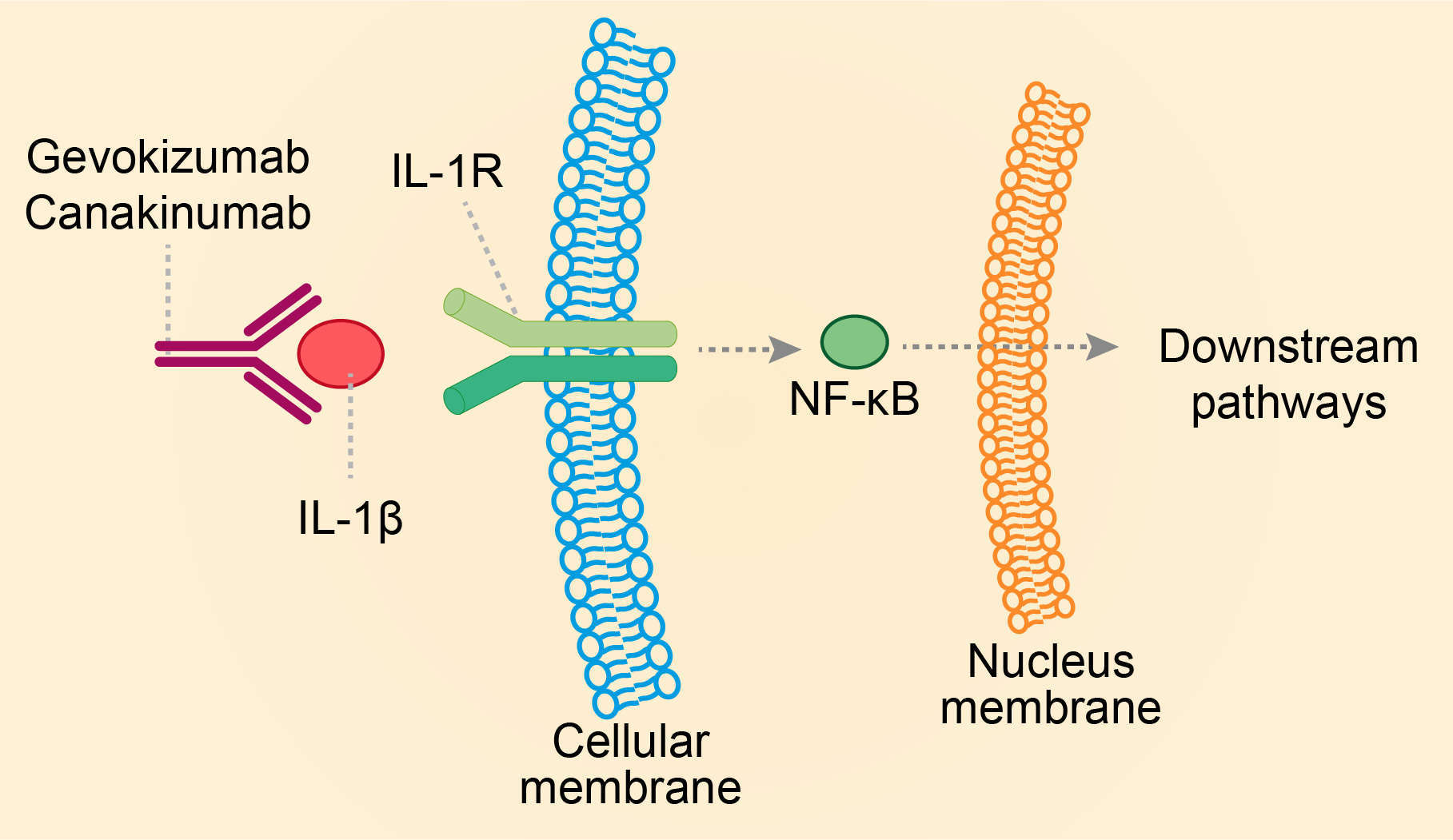 Fig.1 Mechanism of action of Gevokizumab
Fig.1 Mechanism of action of Gevokizumab
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.



