Inotuzumab Ozogamicin Overview
Introduction of Inotuzumab Ozogamicin
Inotuzumab ozogamicin (CMC-544) is an antibody-drug conjugate (ADC) that consists of a derivative of calicheamicin (a potent DNA-binding cytotoxic agent) attached to an engineered humanized monoclonal immunoglobulin G4 (IgG4) antibody targeting CD22. CD22 is a member of a homologous family of sialic-acid-binding immunoglobulin-like lectins (siglecs), which comprise a group of receptors that are restrictedly expressed in immune cells. Siglecs are endocytic receptors where cytotoxic agents conjugated to an antibody can bind and effectively carried into the cell without shedding into the extracellular environment. The therapeutic effect of inotuzumab ozogamicin has initially been shown in non-Hodgkin lymphoma (NHL). In 2017 inotuzumab ozogamicin was approved by the European Medicines Agency and the U.S. Food and Drug Administration (FDA) for the treatment of adults with relapsed or refractory CD22-positive B-cell precursor acute lymphoblastic leukemia (ALL) in 2017.
Mechanism of Action of Inotuzumab Ozogamicin
Inotuzumab ozogamicin consists of a semisynthetic derivative of N-acetyl ɣ-calicheamicin 1, 2-dimethyl hydrazine dichloride (NAc ɣ-calicheamicin DMH), a potent DNA-binding cytotoxic antibiotic, attached to a humanized monoclonal IgG4 antibody, G544, directed against the CD22 antigen present on B cells in all patients with mature B-ALL and most patients (>90%) with precursor B-ALL. Anti-CD22 monoclonal antibody (mAb) without conjugated cytotoxic drug has shown to have no antitumor activity in preclinical models; instead conjugation with cytotoxic agent provided potent dose-depending cellular damage. IgG4 antibodies alone poorly fix complement and therefore cannot cause apoptosis via complement-mediated and antibody-dependent cytotoxicity. Calicheamicin is natural product of Micromon-ospora echinospora and considered to be intolerantly toxic when not bound to the antibody. Calicheamicin is linked to the antibody through 4-(4-acetylphenoxy) butanoic acid (acetyl butyrate), which provides stability in physiologic pH and successful calicheamicin release inside the acidic environment of the lysosomes. Inotuzumab ozogamicin binds to the CD22 receptor on the surface of B cells and the CD22 receptor-inotuzumab ozogamicin complex is internalized forming an endosome. Subsequently, the CD22 receptor-inotuzumab ozogamicin complex containing endosome fuses with lysosomes. This is followed by intracellular release of calicheamicin. Calicheamicin binds to the minor groove of DNA in a sequence specific manner and breaks double-stranded DNA, resulting in cell death.
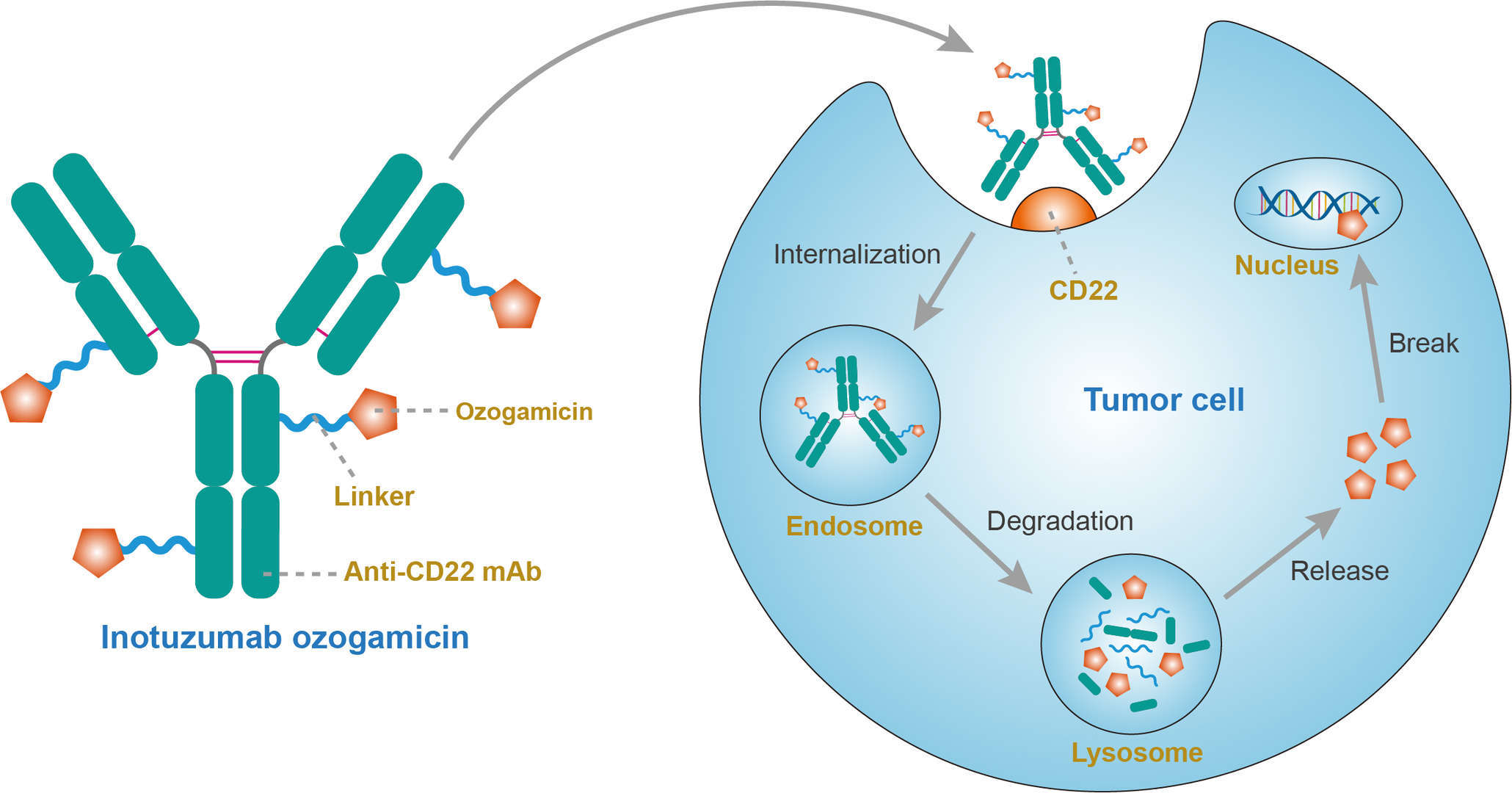 Fig.1 Mechanism of action of inotuzumab ozogamicin
Fig.1 Mechanism of action of inotuzumab ozogamicin
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

