Ixekizumab Overview
Introduction of Ixekizumab
Ixekizumab is a humanized IgG4 monoclonal antibody for the treatment of autoimmune diseases. It was designed specifically to bind interleukin-17A (IL-17A) and produced by recombinant DNA technology in a recombinant mammalian cell line. Ixekizumab has been successively approved for marketing in United States, European Union, Canada, Japan, and Australia, for the treatment of autoimmune diseases mainly including plaque psoriasis and psoriatic arthritis.
Mechanism of Action of Ixekizumab
The etiology of psoriasis is not fully known. But IL-17A, a proinflammatory cytokine produced by T helper (Th) 17 cells, plays a key role in the pathogenesis of plaque psoriasis. IL17A levels are elevated in psoriatic lesions; binding of IL17A to the IL-17 receptors on keratinocytes leads to the release of other proinflammatory mediators, which in turn recruit more Th17 cells, neutrophils, dendritic cells, and lymphoid cells.
By binding to IL-17A, ixekizumab inhibits its binding to the IL-17A receptor, thereby inhibiting its pro-inflammatory downstream effects on keratinocytes.
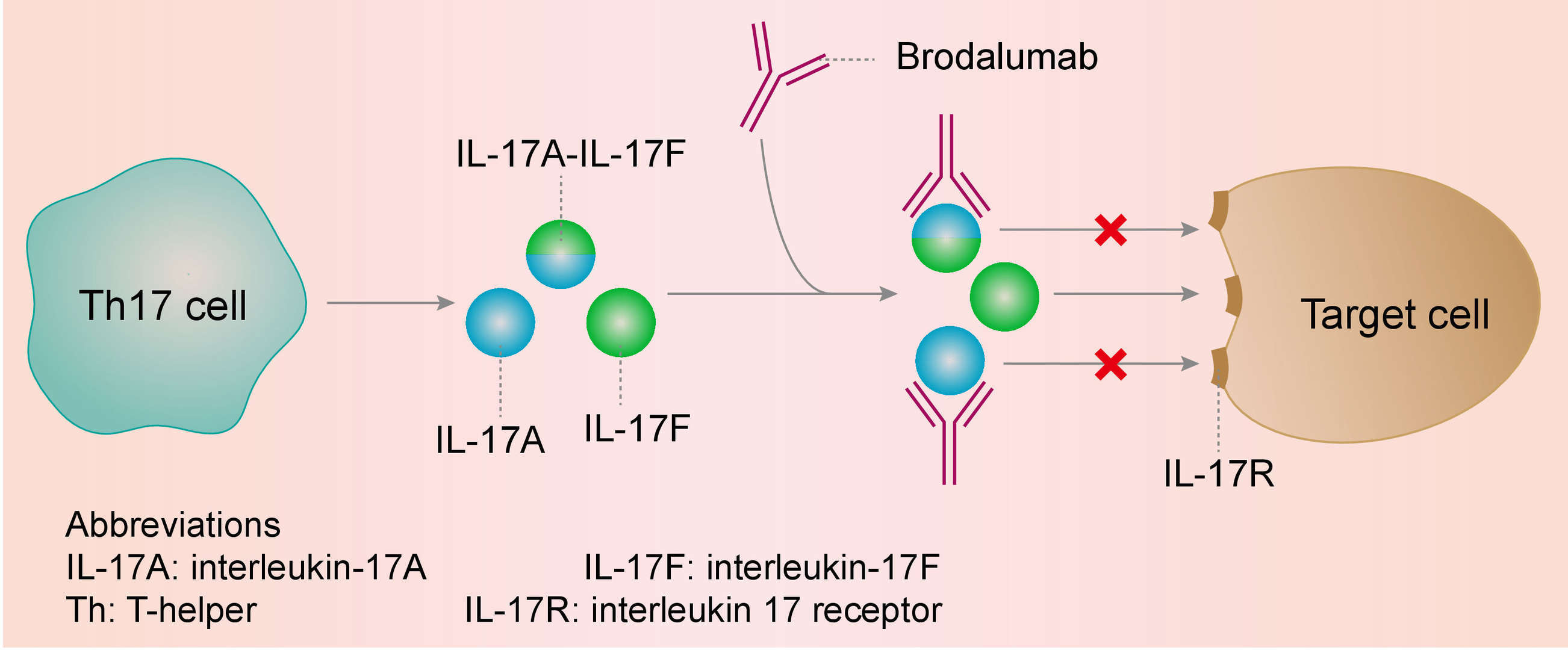 Fig. 1 Mechanism of Action of Ixekizumab
Fig. 1 Mechanism of Action of Ixekizumab
Clinical Projects of Ixekizumab*
| NCT ID | Status | Conditions | Lead Sponsor | Update Time |
| NCT03485976 | Recruiting | Pityriasis Rubra Pilaris | Oregon Health and Science University | April 3, 2018 |
| NCT03099538 | Recruiting | Bullous Pemphigoid, Pemphigoid | Mayo Clinic | April 4, 2017 |
| NCT03073213 | Recruiting | Psoriasis | Eli Lilly and Company | March 8, 2017 |
| NCT03137160 | Recruiting | Pyoderma Gangrenosum | Ohio State University | May 2, 2017 |
| NCT03129100 | Recruiting | Axial Spondyloarthritis | Eli Lilly and Company | April 26, 2017 |
| NCT03073200 | Recruiting | Plaque Psoriasis | Eli Lilly and Company | March 8, 2017 |
| NCT02696798 | Active, not recruiting | Spondyloarthritis | Eli Lilly and Company | March 2, 2016 |
| NCT02757352 | Recruiting | Axial Spondyloarthritis | Eli Lilly and Company | May 2, 2016 |
| NCT03151551 | Recruiting | Psoriatic Arthritis | Eli Lilly and Company | May 12, 2017 |
| NCT02349295 | Active, not recruiting | Psoriatic Arthritis | Eli Lilly and Company | January 28, 2015 |
| NCT02584855 | Active, not recruiting | Psoriatic Arthritis | Eli Lilly and Company | October 23, 2015 |
| NCT02696785 | Active, not recruiting | Spondyloarthritis | Eli Lilly and Company | March 2, 2016 |
| NCT02634801 | Active, not recruiting | Plaque Psoriasis | Eli Lilly and Company | December 18, 2015 |
| NCT03364309 | Recruiting | Plaque Psoriasis | Eli Lilly and Company | December 6, 2017 |
| NCT01646177 | Active, not recruiting | Psoriasis | Eli Lilly and Company | July 20, 2012 |
| NCT01597245 | Active, not recruiting | Psoriasis | Eli Lilly and Company | May 14, 2012 |
| NCT01474512 | Active, not recruiting | Psoriasis | Eli Lilly and Company | November 18, 2011 |
| NCT03168347 | Recruiting | Psoriasis | Wake Forest University Health Sciences | May 30, 2017 |
| NCT03463187 | Recruiting | Moderate-to-severe Chronic Plaque Psoriasis | Jiangsu HengRui Medicine Co., Ltd. | March 13, 2018 |
Approved Drugs of Ixekizumab**
| INN (trade name) | Therapeutic area | Dosage | Strength | Route | Company | Marketing start | Market |
| Taltz | Plaque psoriasis, Psoriatic arthritis, Pustular Psoriasis, Erythrodermic psoriasis | Injection, Solution | 80 mg/mL | Subcutaneous | Eli Lilly Japan K.K. | March 22, 2016 |

|
| Taltz | Moderate-to-severe plaque psoriasis | Injection, Solution | 80 mg/mL | Subcutaneous | Eli Lilly & Co. Ltd. | March 22, 2016 |

|
| Taltz | Active psoriatic arthritis | Injection, Solution | 80 mg/mL | Subcutaneous | Eli Lilly & Co. Ltd. | December 1, 2017 |

|
| Taltz | Plaque psoriasis | Injection, Solution | 80 mg/mL | Subcutaneous | Eli Lilly Nederland B.V. | April 25, 2016 |

|
| Taltz | Psoriatic arthritis | Injection, Solution | 80 mg/mL | Subcutaneous | Eli Lilly Nederland B.V. | January 11, 2018 |

|
| Taltz | Plaque psoriasis, Psoriatic arthritis | Injection, Solution | 80 mg/mL | Subcutaneous | Eli Lilly Canada Inc. | August 11, 2016 |

|
| Taltz | Moderate-to-severe plaque psoriasis | Injection, Solution | 80 mg/mL | Subcutaneous | Eli Lilly Australia Pty Ltd | June 9, 2016 |

|
Resources
* The table was excerpted from the following website
https://clinicaltrials.gov/ct2/results?cond=&term=Ixekizumab
** Information presented in the table were collected from the following websites:
https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=125521
http://www.pmda.go.jp/PmdaSearch/iyakuDetail/GeneralList/3999442G1
http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/003943/human_med_001977.jsp
https://health-products.canada.ca/dpd-bdpp/info.do?lang=en&code=94193
http://search.tga.gov.au/s/search.html?collection=tga-artg&profile=record&meta_i=253893
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

