Lirilumab Overview
Introduction of Lirilumab
Lirilumab (IPH2102/BMS-986015/BMS-986015-01) is a fully human monoclonal antibody (mAb) that is designed to block the interaction between KIR2DL-1,-2,-3 inhibitory receptors and their ligands. A phase 2 clinical trial for acute myeloid leukemia (AML) was terminated early ("failed") in 2017. It was registered for a trial for squamous cell carcinoma of the head and neck (SCCHN), but it may be abandoned. On 22 May 2018, a clinical trial for Bladder Cancer was started.
Mechanism of Action of Lirilumab
Natural killer (NK) cells are critical effectors of the innate immune system; they are regulated by a balance of signaling from activating and inhibitory receptors and possess potent anticancer effects in a variety of tumor models. Therefore, quantitative or functional alterations of NK cells might contribute to cancer progression. For example, NK cell number and function correlate with relapse-free survival in acute myeloid leukemia (AML). NK cell activation is partially controlled by KIRs upon binding with their ligands. KIRs constitute a diverse family of activating and inhibitory checkpoint receptors that prevent NK cell activation upon binding with their ligands (primarily human leukocyte antigen-C, HLA-C, molecules). Distinct KIR family members bind to different major histocompatibility complex (MHC) class I allotypes. The clinical relevance of KIR inhibition has been shown in allogeneic haplo-mismatched stem cell transplantation (alloSCT) in patients with AML. Mismatches between KIRs on donor NK cells and recipient MHC class I molecules enable NK cell activation, which is associated with improved relapse-free survival and overall survival. The results of these stem-cell transplantation studies suggest that, in the absence of KIR interactions with MHC class I molecules, alloreactive NK cells may eradicate residual leukemia. Based on these findings, it can be expected that anti-KIR monoclonal antibodies (mAbs) may enhance the antitumor responses of natural killer (NK) cells. Blocking these receptors facilitates activation of NK cells and, potentially some subsets of T cells, ultimately leading to destruction of tumor cells. At early stage, IPH2101, a fully human anti-KIR IgG4 mAb, is produced from human hybridoma cells. To increase yield and avoid the formation of half -antibodies described with human IgG4, a recombinant version, lirilumab (IPH2102/BMS-986015/BMS-986015-01), was developed. Lirilumab (IPH2102) is a fully human IgG4 monoclonal antibody produced by recombinant Chinese hamster ovary cells. Lirilumab recognizes the same epitope as IPH2101. It has the same primary amino acid sequence as IPH2101, except for one mutation introduced in the constant region of the heavy chain, where a serine is substituted for a proline. This mutation leads to slight changes in the glycosylation of the antibody. Lirilumab has the same mechanism of action as IPH2101 and therefore similar pharmacologic properties are expected.
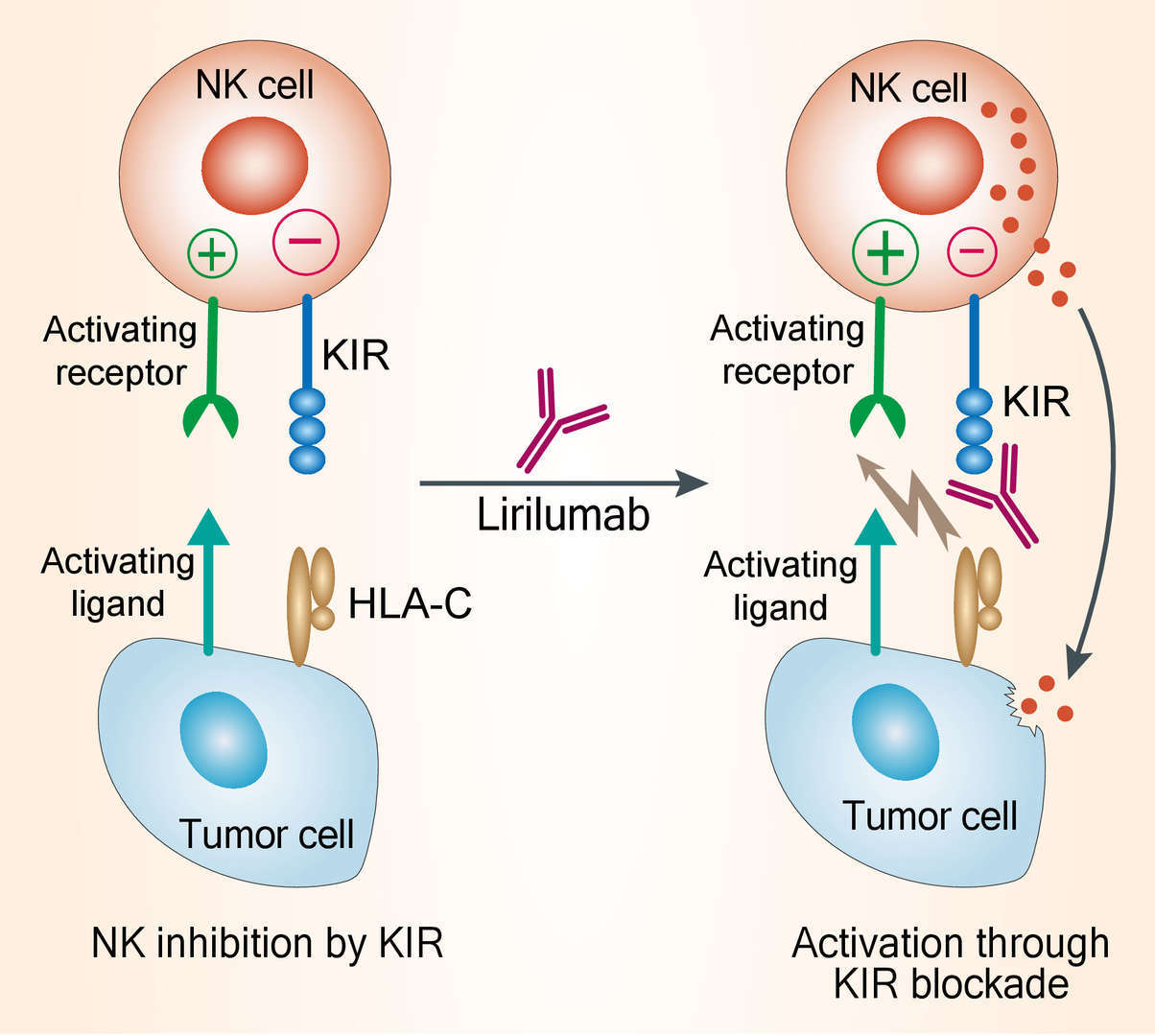 Fig.1 Mechanism of action of lirilumab
Fig.1 Mechanism of action of lirilumab
Clinical Projects of Lirilumab*
| NCT ID | Status | Conditions | Lead Sponsor | Update Time |
| NCT03532451 | Not yet recruiting | Bladder Cancer | PrECOG, LLC. | May 22, 2018 |
| NCT02481297 | Active, not recruiting | Leukemia, Chronic Lymphocytic Leukemia, Lymphocytic Leukemia | M.D. Anderson Cancer Center | June 25, 2015 |
| NCT02599649 | Active, not recruiting | Leukemia | M.D. Anderson Cancer Center | November 6, 2015 |
| NCT02399917 | Active, not recruiting | Leukemia | M.D. Anderson Cancer Center | March 26, 2015 |
| NCT03341936 | Recruiting | Squamous Cell Carcinoma of the Head and Neck | Dana-Farber Cancer Institute | November 14, 2017 |
| NCT03203876 | Active, not recruiting | Advanced Cancer | Bristol-Myers Squibb | June 29, 2017 |
| NCT01714739 | Active, not recruiting | CANCER,NOS | Bristol-Myers Squibb | October 26, 2012 |
| NCT03347123 | Recruiting | Solid Tumors | Incyte Corporation | November 20, 2017 |
* The table was excerpted from the following website
https://clinicaltrials.gov/ct2/results?cond=&term=Lirilumab
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

