Matuzumab Overview
Introduction of Matuzumab
Matuzumab (formerly EMD 72000) is a humanized IgG1 anti- epidermal growth factor receptor (EGFR) monoclonal antibody (mAb) produced by molecularly splicing the anti-EGFR binding regions of its murine predecessor (known as mAb425) to human IgG1 variable and constant regions. It contains less than 5 percent of the original murine content, retains high affinity for human EGFR, and has no reactivity with murine EGFR.
Mechanism of Action of Matuzumab
Four mechanisms have been proposed for the antitumor effects of matuzumab and other EGFR-targeting mAbs. First, because therapeutic EGFR-targeting monoclonal antibodies have a higher affinity for EGFR than do the receptor’s naturally occurring ligands, these antibodies inhibit natural ligand binding to the receptor. This blocks TK activation of EGFR, the first step in triggering the mitogenic intracellular signaling cascades through the Ras-Raf-MAPK, and PI-3 kinase/Akt transduction pathways that stimulate the cell to grow, divide, and move. According to the second proposed mechanism, binding of antibodies to EGFR downregulates expression of EGFR by removing the receptor from the surface of the tumor cell through a process of endocytosis and intracellular degradation. This prolongs the period during which the tumor cell is unable to respond to extracellular growth factor ligands, and it may be an important difference between the antitumor effects of EGFR antibodies and those of agents such as gefitinib and erlotinib, which block receptor activation by competitively inhibiting EGFR enzymatic activity (TK) but have not been shown to affect receptor expression. There is little supporting evidence that anti-EGFR antibodies operate through the third mechanism, inhibition of receptor dimerization, though this is the proposed mechanism for other ErbB-family targeting antibodies, as discussed above. It is more likely that anti-EGFR antibodies inhibit EGFR dimerization secondary to inhibition of ligand binding. A fourth mechanism of action of immunoglobulin G1(IgG1) anti-EGFR antibodies is well supported in preclinical studies but is difficult to demonstrate in patients or animal models. This stimulation of the immunological response is termed antibody dependent cell-mediated cytotoxicity (ADCC). ADCC occurs only with the IgG1 subtype. It occurs when an IgG1 antibody bound to a cell is recognized by lymphocyte effector cells with receptors for the IgG antibody, thereby inducing the death of the target cell through well-described mechanisms of immune cell–mediated cytotoxicity. ADCC effector cells are monocytes and natural killer lymphocytes.
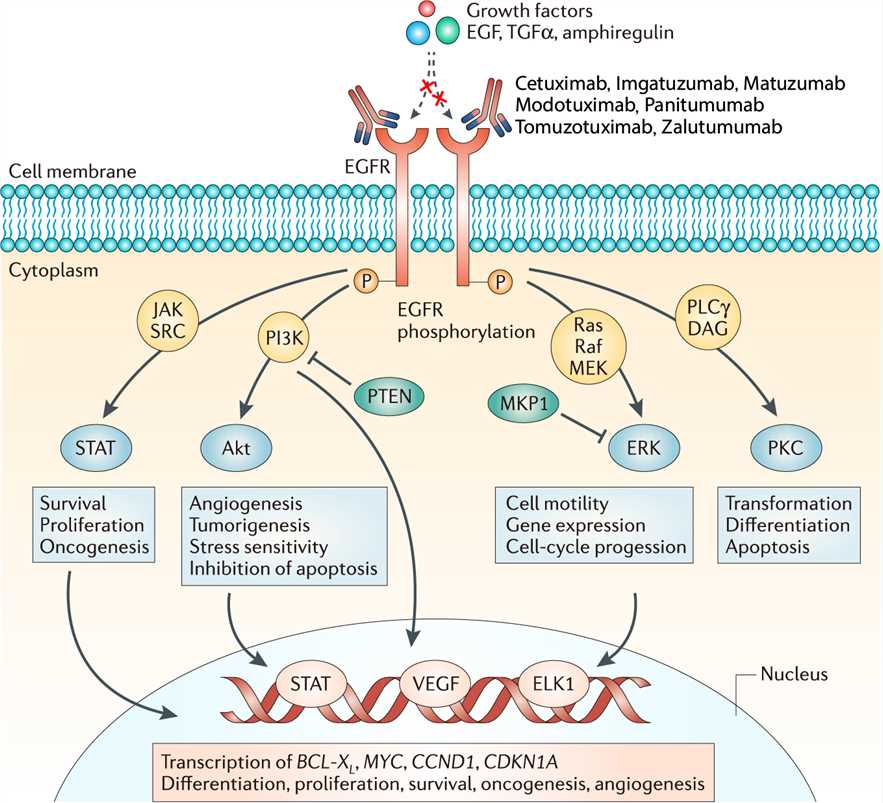
Fig.1 Mechanism of action of Matuzumab
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.



