Namilumab Overview
Introduction of Namilumab
Namilumab (alternative identifier MT203) is a human monoclonal antibody (class IgG1 kappa) that targets granulocyte macrophage-colony stimulating factor (GM-CSF), which is also known as colony stimulating factor 2 (CSF2). It is currently being researched for application in rheumatoid arthritis (RA) and psoriatic arthritis. Clinical trials investigating the therapeutic utility of Namilumab have include phase I and phase II clinical trials to establish the safety, tolerability and preliminary therapeutic utility of the antibody in plaque psoriasis and rheumatoid arthritis.
Mechanism of Action of Namilumab
Granulocyte macrophage colony-stimulating factor (GM-CSF) is a hematopoietic growth factor produced by a number of different cell types, including: T cells, macrophages, mast cells, endothelial cells, smooth muscle cells, epithelial cells, and fibroblasts. GM-CSF stimulates the proliferation and activation of mature myeloid cells inducing the production of inflammatory molecules, thereby acting as a pro-inflammatory cytokine. As GM-CSF is a key activator of the innate immune system, it is likely to play an important role in the pathogenesis of autoimmune inflammatory diseases (including RA) in which macrophages, neutrophils, granulocytes, eosinophils, and dendritic cells contribute to disease progression. In patients with RA, GM-CSF is aberrantly overproduced; GM-CSF levels are moderately elevated in the plasma and highly elevated in the synovial fluid, particularly in the pannus at sites of cartilage erosion. The contribution of GM-CSF to the development of RA has also been documented in various in vitro and in vivo mouse models.
The basic GM-CSF receptor subunit consists of a unique a-chain and a common b-chain. However, unusually for class I cytokine receptors, the crystal structure of the ligand-bound human GM-CSF receptor has a higher order assembly comprising both an a hexamer, consisting of two molecules each of ligand, the receptor a chain and b chain, as well as a dodecamer in which two hexameric complexes associate. This structure has enabled a sequential model of GM-CSF receptor activation to be put forward in line with a dose-dependent utilization of receptor activation sites and signaling pathways to help explain the different biologic responses occurring at increasing GM-CSF concentrations in myeloid populations, namely survival, proliferation, and activation/differentiation. As regards the downstream signaling pathways, a key transcription factor is signal transducer and activator of transcription (STAT) 5 which is phosphorylated in turn by Janus kinase 2. Other signaling molecules/pathways involved in GM-CSFdependent myeloid cell and DC development/function have reported to be mitogen-activated protein kinase, nuclear factor (NF)-kB, and phosphoinositide 3-kinase-Akt. GM-CSF has been considered a growth and survival factor for several leukemias including acute myeloid leukemia, chronic myeloid leukemia, and juvenile myelomonocytic leukemia (JMML). In vitro hypersensitivity to GM-CSF is one of the criteria for the diagnosis of JMML and results from continuous activation of a GM-CSFR/RAS/ERK signal transduction pathway.
Namilumab targets and neutralizes circulating GM-CSF, inhibiting GM-CSF-involved immune response, including the recruitment of myeloid cells to a tumor, leukocyte differentiation, autoimmunity and inflammation.
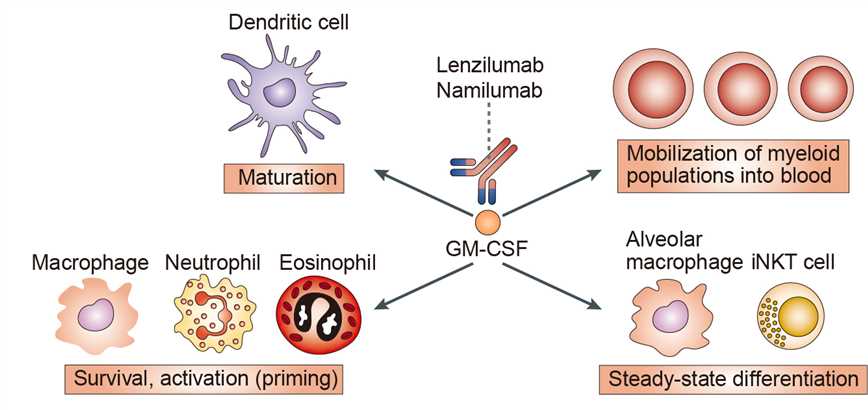
Fig 1. Mechanism of Action of Namilumab
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.



