Nivolumab Overview
Introduction of Nivolumab
Nivolumab (BMS-936558, ONO-4538, or MDX1106) is a human immunoglobulin G4 (IgG4) monoclonal antibody (mAb). The gamma 1 heavy chain is 91.8% unmodified human design while the kappa light chain is 98.9%. It is the first-in-human programmed death-1 (PD-1) immune checkpoint inhibitor antibody that disrupts the interaction of the PD-1 receptor with its ligands programmed death-ligand 1 (PD-L1) and programmed death-ligand 2 (PD-L2), thereby inhibiting the cellular immune response.Nivolumab received Food and Drug Administration (FDA) approval for the treatment of melanoma in December 2014. In April 2015, the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) recommended approval of Nivolumab for metastatic melanoma as a monotherapy. In March 2015, the US FDA approved it for the treatment of squamous cell lung cancer (SCLC). On 19 June 2015 the European Commission granted a marketing authorization valid throughout the European Union. In November 2015, the FDA approved nivolumab as a second-line treatment for renal cell carcinoma (RCC) after having granted the application breakthrough therapy designation, fast track designation, and priority review status. In May 2016, the FDA approved nivolumab for the treatment of patients with classical Hodgkin lymphoma (cHL) who have relapsed or progressed after autologous hematopoietic stem cell transplantation (auto-HSCT) and post-transplantation brentuximab vedotin. On December 20, 2017, the FDA granted approval to nivolumab for adjuvant treatment of melanoma with involvement of lymph nodes or for metastatic disease with complete resection. On April 16, 2018, the FDA granted approval to nivolumab in combination with ipilimumab for the first-line treatment of intermediate and poor risk advanced RCC patients. Side effects include severe immune-related inflammation of the lungs, colon, liver, kidneys, and thyroid, and there are effects on skin, central nervous system, the heart, and the digestive system.
Mechanism of Action of Nivolumab
PD-1 is a member of the CD28 family of T-cell receptors, and is primarily expressed on activated T cells, B cells and myeloid cells. PD-1 is upregulated on T cells after chronic antigen stimulation, and is a marker of T-cell exhaustion. Key ligands for PD-1 include PD-L1 (B7-H1) and PD-L2 (B7-DC), which are expressed by immune cells, and can also be induced on a variety of tissues, particularly after exposure to inflammatory cytokines, such as IFN-γ. PD-L1 is expressed on a variety of human cancers, and this expression is thought to play an important role in evading antitumor immune responses. Human tumors have also been documented to express PD-L2, although its role is less clear. When PD-1 on T cells binds PD-L1 or PD-L2, the T cell receives an inhibitory signal and no longer mounts productive immune responses. Nivolumab blocks PD-L1 from binding to PD-1, allowing the T cell to work.
> 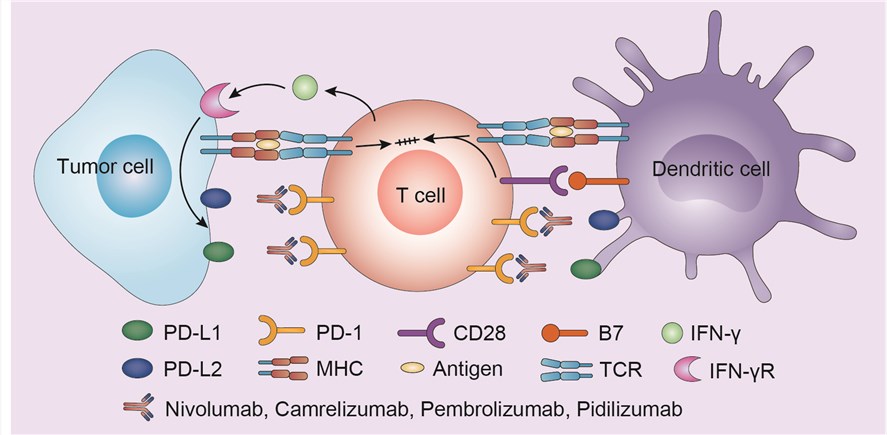 Fig.1 Mechanism of action of nivolumab
Fig.1 Mechanism of action of nivolumab
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

