Olaratumab Overview
Introduction of Olaratumab
Olaratumab (IMC-3G3) is a fully human IgG1κ monoclonal antibody developed by Eli Lilly and Company. It selectively binds the external domain of human platelet-derived growth factor receptor (PDGFR)-α. Olaratumab has been approved by USA, Canada, and European Union to use for the treatment of adults with advanced soft-tissue sarcoma (STS) who cannot be cured by cancer surgery or radiation therapy.
Mechanism of Action of Olaratumab
Platelet-derived growth factor (PDGF) and PDGFR together comprise the PDGF/PDGFR signaling axis, which plays a crucial role in the normal biological downstream cellular signaling required for organ development and function, in addition to promoting wound healing. Inadvertently, the PDGF/ PDGFR axis can promote autocrine growth stimulation of cancer cells. As a type of PDGFR, PDGFRα was found to be a receptor tyrosine kinase expressed in STS and its activation has been implicated in aberrant cell signaling leading to tumor growth and metastasis. As a PDGFRα antagonist, olaratumab binds to PDGFRα with high specificity and affinity, thereby, blocking PDGF-AA, PDGF-BB, and PDGFCC receptor activation and downstream signaling.
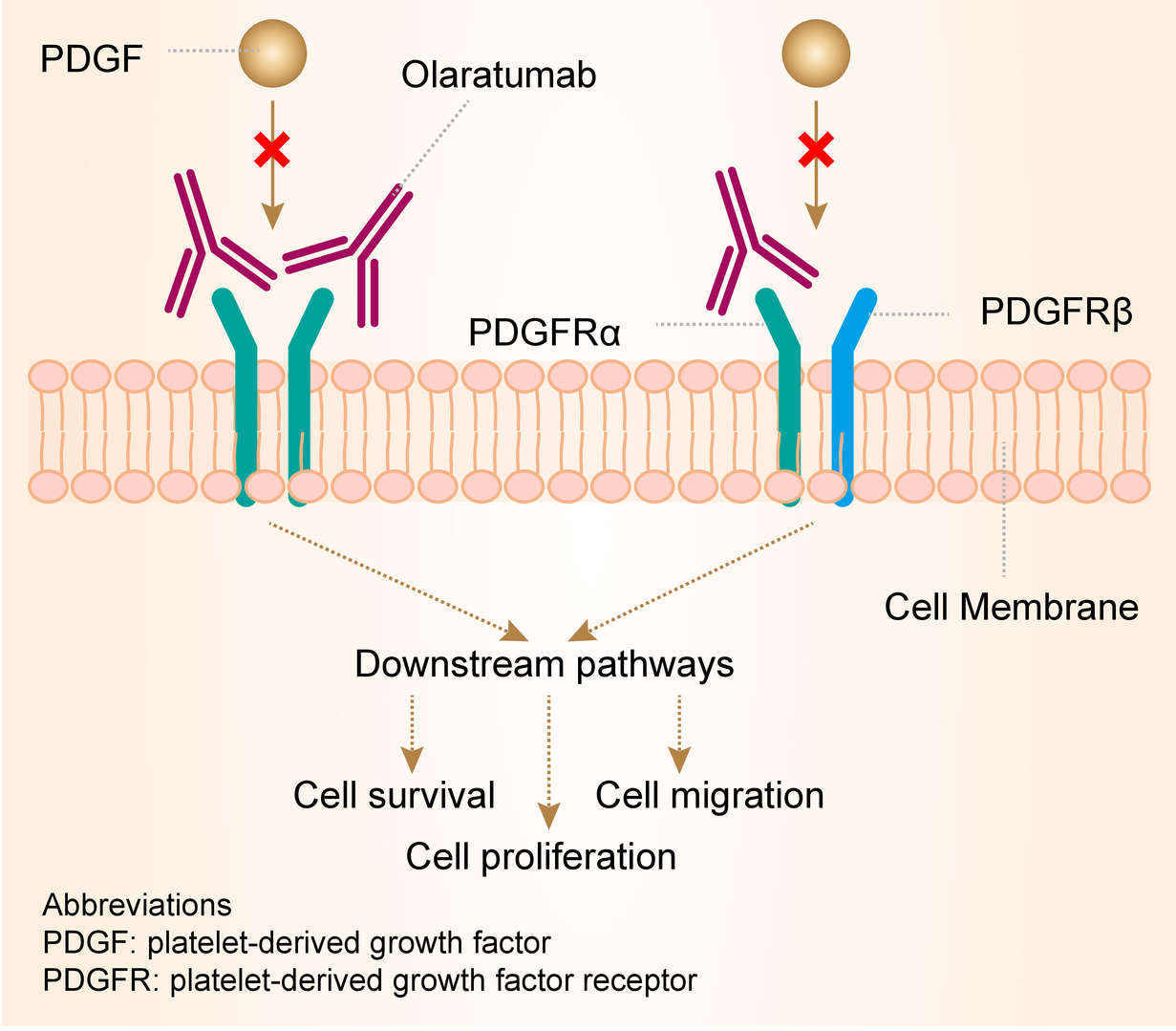 Fig.1 Mechanism of Action of Olaratumab
Fig.1 Mechanism of Action of Olaratumab
Clinical Projects of Olaratumab*
| NCT ID | Status | Conditions | Lead Sponsor | Update Time |
| NCT02377752 | Active, not recruiting | Neoplasm | Eli Lilly and Company | March 4, 2015 |
| NCT03437070 | Not yet recruiting | Leiomyosarcoma | Marilyn Huang | February 19, 2018 |
| NCT03283696 | Recruiting | Soft Tissue Sarcoma | Eli Lilly and Company | September 14, 2017 |
| NCT02783599 | Active, not recruiting | Soft Tissue Sarcoma | Eli Lilly and Company | May 26, 2016 |
| NCT02659020 | Recruiting | Soft Tissue Sarcoma | Eli Lilly and Company | January 20, 2016 |
| NCT03126591 | Recruiting | Soft Tissue Sarcoma | Eli Lilly and Company | April 24, 2017 |
| NCT02677116 | Recruiting | Neoplasm Metastasis | Eli Lilly and Company | February 9, 2016 |
| NCT02584309 | Recruiting | Soft Tissue Sarcoma, Undifferentiated Pleomorphic Sarcoma, Leiomyosarcoma, Liposarcoma, Synovial Sarcoma, Myxofibrosarcoma, Angiosarcoma, Fibrosarcoma, Malignant Peripheral Nerve Sheath Tumor, Epithelioid Sarcoma | Washington University School of Medicine | October 22, 2015 |
| NCT03086369 | Recruiting | Metastatic Pancreatic Cancer | Eli Lilly and Company | March 22, 2017 |
| NCT02451943 | Active, not recruiting | Soft Tissue Sarcoma | Eli Lilly and Company | May 22, 2015 |
| NCT00918203 | Active, not recruiting | Non-Small Cell Lung Cancer | Eli Lilly and Company | June 11, 2009 |
Approved Drugs of Olaratumab**
| INN (trade name) | Therapeutic area | Dose | Strength | Route | Company | Marketing start | Market |
| Lartruvo | Soft tissue sarcoma (STS) | Injection, Solution | 10 mg/mL | Intravenous | Eli Lilly and Company | October 19, 2016 |

|
| Lartruvo | Soft tissue sarcoma (STS) | Injection, Solution | 10 mg/mL | Intravenous | Eli Lilly Nederland B.V. | September 11, 2016 |

|
| Lartruvo | Soft tissue sarcoma (STS) | Injection, Solution | 10 mg/mL | Intravenous | Eli Lilly Canada Inc. | December 22, 2017 |

|
Resources
* The table was excerpted from the following website
https://clinicaltrials.gov/ct2/results?cond=&term=Olaratumab
** Information presented in the table were collected from the following websites:
https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=761038
https://health-products.canada.ca/dpd-bdpp/info.do?lang=en&code=95790
https://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/004216/human_med_002036.jsp
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.



