Otelixizumab Overview
Introduction of Otelixizumab
Otelixizumab, also known as TRX4, is a monoclonal antibody (mAb) that can bind to a receptor found on all T cells called CD3, which is involved in normal T cell signaling. It has been genetically engineered to remove the glycosylation site in the Fc domain and this limits its ability to bind to complement or Fc receptors and reduces the risk of adverse clinical reactions due to cytokine release. As a monoclonal antibody, otelixizumab consists of two heavy chains and two light chains. The heavy chains are humanized γ1 (gamma-1) chains from rats, making otelixizumab an immunoglobulin G1. The light chains are chimeric human/rat λ (lambda) chains. Otelixizumab is believed to inhibit the function of autoreactive T cells, which are important in propagating autoimmune diseases, while inducing regulatory T cell pathways that promote immunological tolerance and inhibit autoreactive disease activity.
Mechanism of Action of Otelixizumab
Autoimmune diseases are triggered by autoreactive T and B cells that escape mechanisms of immune tolerance. Type 1 diabetes (T1D) is an organ-specific autoimmune disease characterized by the selective destruction of pancreatic β-cells. The histopathology of T1D is defined by a decreased β-cell mass with infiltration of mononuclear cells into the islets of Langerhans. T1D is often complicated with other autoimmune diseases, and anti-islet autoantibodies precede the clinical onset of disease. Otelixizumab downregulates pathogenic T cells and upregulates T regulatory cells, thus inhibiting the autoimmune process responsible for type 1 diabetes. The potential utility of otelixizumab in the management of type 1 diabetes has been demonstrated in animal and human studies. Data suggest that the drug works by blocking the function of effector T cells, which mistakenly attack and destroy insulin-producing beta cells, while stimulating regulatory T cells, which are understood to protect against effector T cell damage, thus preserving the beta cells' normal ability to make insulin. Binding of Otelixizumab to the CD3/TCR complex leads to antigenic modulation, at the same time Otelixizumab-induced signaling through the CD3/TCR complex can render the T cell anergic or trigger apoptosis. While antigenic modulation and energy only render lymphocytes ignorant to antigen and lead to transient immunosuppression, Otelixizumab-induced tolerance is dependent on apoptosis. Apoptotic T cells and macrophages that ingest the apoptotic bodies both produce TGF-β that promotes a tolerogenic microenvironment. TGF-β can induce FoxP3 in CD4+ T cells, rendering them suppressive. Both, TGF-β and CD4+FoxP3+ T cells inhibit effector T cells and skew antigen presenting cells such as dendritic cells toward a tolerogenic phenotype.
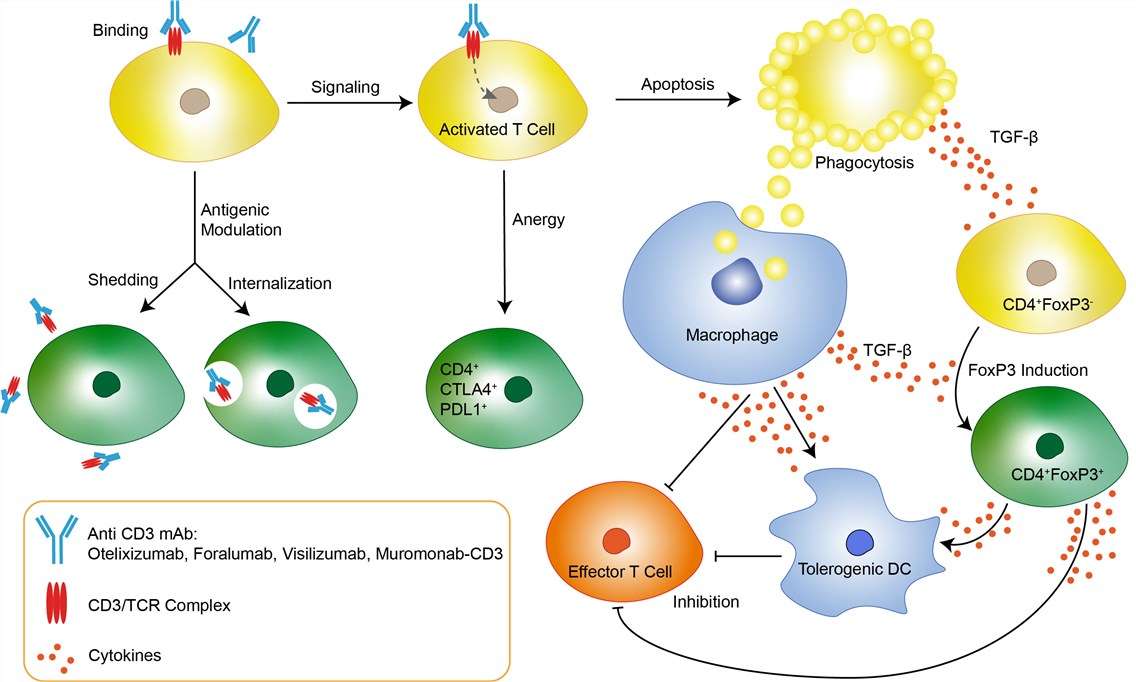 Fig 1. Mechanism of Action of Otelixizumab
Fig 1. Mechanism of Action of Otelixizumab
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

