Panitumumab Overview
Introduction of Panitumumab
Panitumumab, formerly ABX-EGF, is a fully human monoclonal antibody (mAb) specific to the epidermal growth factor receptor (also known as EGF receptor, EGFR, ErbB-1 and HER1 in humans). This methodology is based on inactivating the mouse immunoglobulin genes that are replaced by a megabase gene containing the human heavy and κ chains. The result is the generation of fully human antibodies that do not contain murine portions of the IgG molecule as chimeric antibodies do. This property avoids the formation of human antimouse antibodies, which may result in more frequent hypersensitivity reactions and shorter half-life.
Panitumumab was approved by the U.S. Food and Drug Administration (FDA) for the first time in September 2006, for the treatment of EGFR-expressing metastatic colorectal cancer with disease progression despite prior treatment. Panitumumab was approved by the European Medicines Agency (EMEA) in 2007, and by Health Canada in 2008 for the treatment of refractory EGFR-expressing metastatic colorectal cancer in patients with non-mutated (wild-type) KRAS. In July 2009, the FDA updated the labels of two anti-EGFR mAb drugs (panitumumab and cetuximab) indicated for the treatment of metastatic colorectal cancer to include information about KRAS mutations.
Mechanism of Action of Panitumumab
EGFR, a tyrosine kinase transmembrane receptor belonging to the ErbB family, plays a crucial role in cell proliferation, survival, and differentiation in many malignancies due to EGFR dysregulation. Many ligands, in addition to epidermal growth factor (EGF), are involved in EGFR activation, including transforming growth factor alpha, amphiregulin, betacellulin, epigen, epiregulin, and heparin-binding EGF. These various ligands are capable of binding to the extracellular ligand-binding domain to activate downstream mechanisms for tumor development and proliferation. EGFR activation leads to several signaling pathways, including RAS/RAF/MEK/ERK, PI3CK/AKT/mTOR, Src family kinases, STATs, and PLCγ-PKC, thus leading to cell survival through tumor growth, angiogenesis, tumor invasion, and migration. EGFR is overexpressed in certain human cancers, including colon and rectal cancer. Panitumumab binds specifically to the extracellular domain of EGFR on both normal and tumor cells, competitively inhibiting the binding of other ligands to EGFR and thus preventing receptor phosphorylation and subsequent activation of downstream signaling cascade. This results in inhibition of cell growth, induction of apoptosis, decreased proinflammatory cytokine and vascular growth factor production, and EGFR downregulation through receptor internalization.
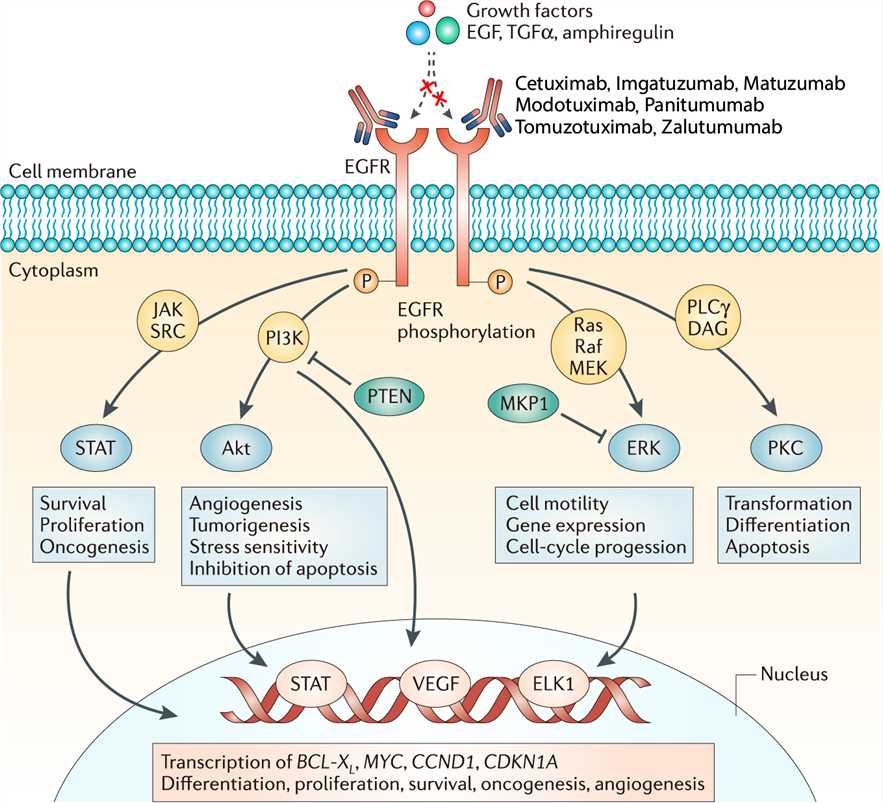
Fig.1 Mechanism of action of Panitumumab
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

