Pinatuzumab Vedotin Overview
Introduction of Pinatuzumab Vedotin
Pinatuzumab vedotin (DCDT2980S, FCU2703) is an anti-CD22 antibody-drug conjugate (ADC) developed for the treatment of diffuse large B-cell lymphoma (DLBCL). It was designed by link an anti-CD22 monoclonal antibody (mAb) and a potent microtubule-disrupting agent monomethyl auristatin E (MMAE) - via a protease sensitive maleimidocaproyl-valine- citrulline- p-aminobenzoyloxycarbonyl (MC-vcPAB) linker. The linker to the monoclonal antibody is stable in extracellular fluid but is cleaved by cathepsin once the conjugate has entered a tumour cell, thus activating the antimitotic mechanism. MMAE is a potent antimitotic drug derived from peptides occurring in marine shell-less mollusc Dolabella auricularia called dolastatins which show potent activity in preclinical studies, both in vitro and in vivo, against a range of lymphomas, leukemia and solid tumors. Pinatuzumab vedotin has proved its efficacy in inducing a complete tumor regression in xenograft lymphoma models. In addition, it was well tolerated and more effective as a single agent compared to standard immunochemotherapy. These findings warranted further research in humans.
Mechanism of Action of Pinatuzumab Vedotin
A CD22 (Siglec-2) antigen is responsible for the regulation of B lymphocyte function, by binding lectin derivatives; it belongs to the sialic acid-binding immunoglobulin-type lectins (SIGLECS) family of lectins predominantly expressed in B cells. Ig domains 1 and 2 of murine CD22 constitute the ligand-binding domain and bind multiple sialylated ligands expressed on B and T cells. CD22 has been found to be expressed on most B-lymphocyte lineage cells, including lymphoblasts. It forms part of the B cell receptor (BCR) and therefore regulates B-cell functions, such as immunoglobulin production. The fact that it is internalized upon ligation with a specific antibody together with its unique expression have made it a particularly valuable target for ADCs in the treatment of B-cell malignancies. Once pinatuzumab vedotin binds to the CD22 and becomes internalized in the tumor cells, the MC-vcPAB linker will be degraded by proteases, resulting in the release of the MMAE drug, which is an antimitotic agent inhibiting cell division by blocking the polymerization of tubulin.
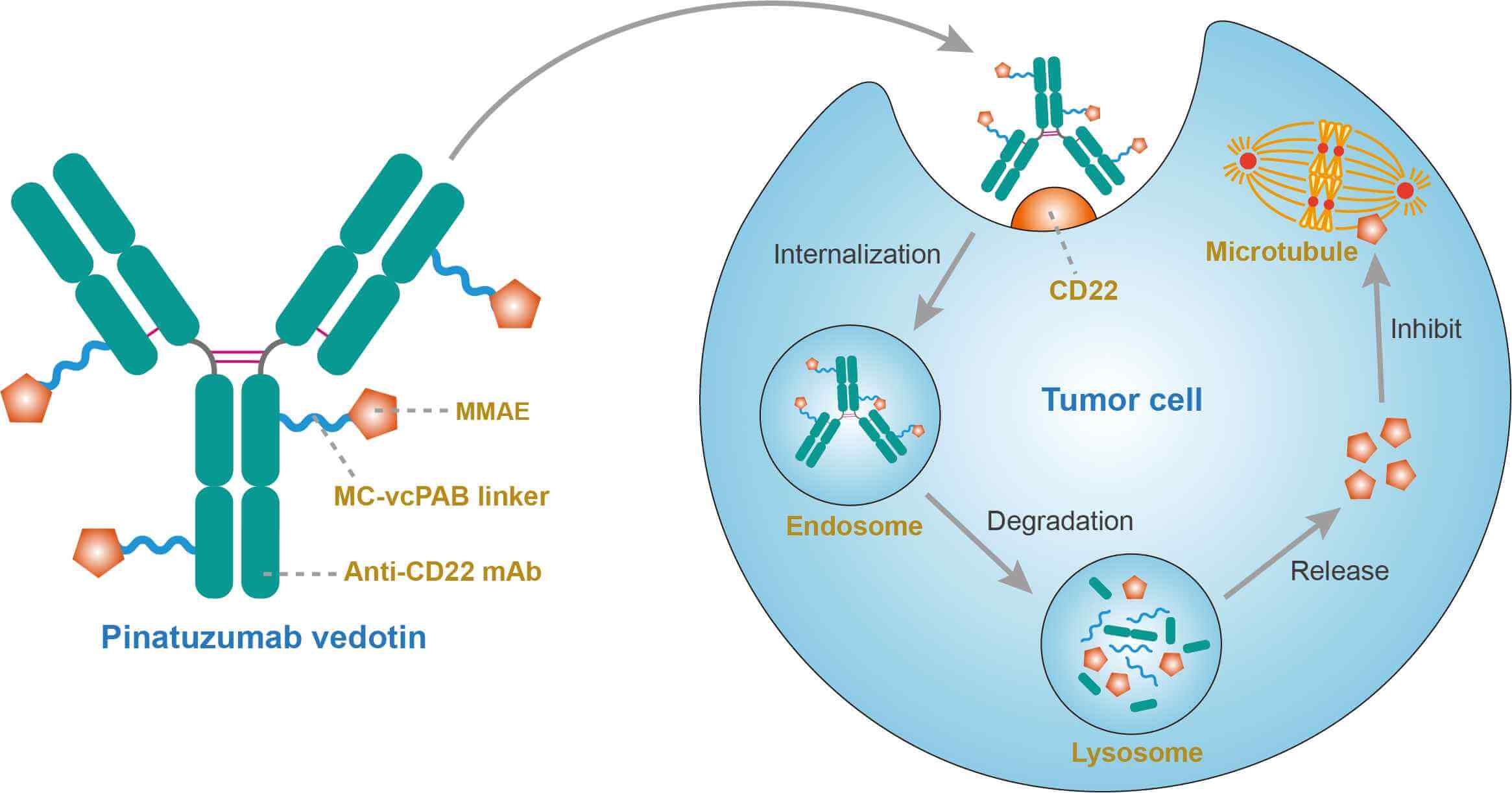 Fig.1 Mechanism of action of Pinatuzumab Vedotin
Fig.1 Mechanism of action of Pinatuzumab Vedotin
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.



