Raxibacumab Overview
Introduction of Raxibacumab
Raxibacumab is a recombinant human IgG1λ monoclonal antibody (mAb) intended for the prophylaxis and treatment of inhaled anthrax. It blocks the binding of protective antigen (PA) to its cell receptor. Its efficacy has been proven in rabbits and monkeys. In December 2012, the United States Food and Drug Administration (FDA) approved raxibacumab for treatment of and prophylaxis against inhalational anthrax. Its labeled uses are to treat inhalational anthrax in combination with appropriate antibacterial drugs, and for prophylaxis when alternative therapies are not appropriate or available. For its approval, the FDA granted fast track designation, priority review, orphan product designation, and applied the Animal Efficacy Rule. Under this rule, medications are given FDA approval based on efficacy findings from adequate and well controlled animal studies when it is not feasible or ethical to conduct studies in human subjects.
Mechanism of Action of Raxibacumab
Anthrax is caused by the spore-forming bacterium Bacillus anthracis and can manifest in three forms cutaneous gastrointestinal, and inhalational. Bacillus anthracis is a large rod-shaped, aerobic, Gram-positive, spore-forming bacterium that exists in a spore or vegetative state. The anthrax toxin is composed of two binary combinations, each containing a common binding component known as protective antigen (PA). The other two components, edema factor and lethal factor, are enzymes. PA combines with edema factor to form edema toxin, and in a similar way with lethal factor to form lethal toxin. PA is a protein that mediates binding to its receptors in the cell membrane of host cells. Binding to either a high-affinity or low-affinity receptor (ANTXR1/2) that may or may not require a coreceptor (LRP6) occurs, with subsequent transformation of PA, resulting in pore formation and facilitating translocation of edema factor and lethal factor into the cell cytosol. PA is therefore essential for intracellular translocation of both edema and lethal toxins. PA induces immunization, and all current acellular or attenuated live anthrax vaccines contain or express PA. Raxibacumab binds to PA at the domain IV epitope with an affinity of 2.78±0.9nM, and its inhibition is dose-dependent. Raxibacumab does not have any direct antibacterial activity. Therefore, it is advised that its use should be combined with the antibiotics recommended for the treatment of anthrax.
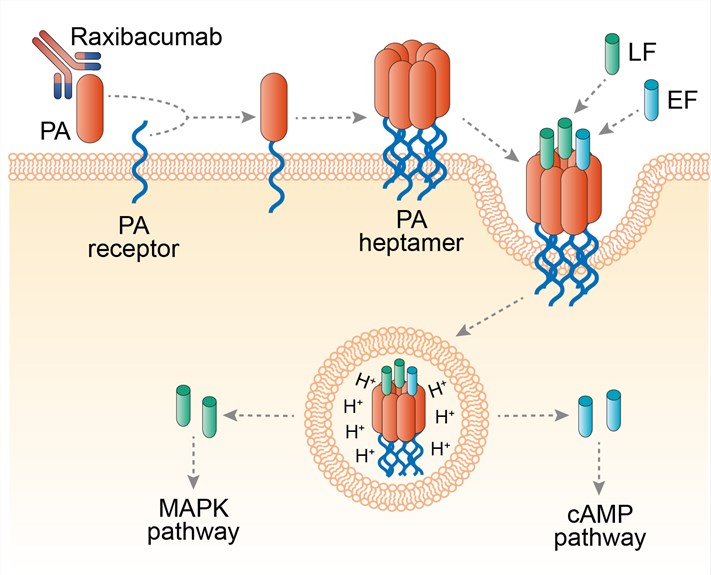 Fig.1 Mechanism of action of raxibacumab
Fig.1 Mechanism of action of raxibacumab
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.



