Secukinumab Overview
Introduction of Secukinumab
Secukinumab is a fully human IgG1/κ-class monoclonal antibody (mAb) composed of 215 light chain amino acids and 457 heavy chain amino acids. It was discovered and developed by Novartis with the developmental name AIN457, for the treatment of uveitis, rheumatoid arthritis, ankylosing spondylitis, and psoriasis. It is produced in Chinese Hamster Ovary (CHO) cells using recombinant DNA technology.
It binds to and neutralises the proinflammatory cytokine interleukin-17A (IL-17A). In January 2015, the U.S. Food and Drug Administration (FDA) approved secukinumab to treat adults with moderate-to-severe plaque psoriasis. It was the first IL17A inhibiting drug ever approved. In January 2016, the FDA approved it to treat adults with ankylosing spondylitis, and psoriatic arthritis and in February 2018 a label update was approved to include the treatment for moderate-to-severe scalp psoriasis. Till now, secukinumab has gained the approval for marketing in USA, Europe Union, Canada, Japan, and Australia for the treatment of psoriasis, ankylosing spondylitis, and psoriatic arthritis. It is given by subcutaneous injection and is sold in a pre-filled syringe or autoinjector that can be used at home and as a lyophilized powder for use in hospitals and clinics.
Mechanism of Action of Secukinumab
Secukinumab works by targeting IL-17A and inhibiting its interaction with the IL-17 receptor. IL-17A can be produced by a range of different cell types as part of both the adaptive and innate immune responses, including neutrophils, macrophages, mast cells, Group 3 innate lymphoid cells, T helper 17 (Th17) cells and cytotoxic T cells. IL-17A is a naturally occurring cytokine that is involved in normal inflammatory and immune responses. IL-17A plays a key role in the pathogenesis of plaque psoriasis, psoriatic arthritis and ankylosing spondylitis and is up-regulated in lesional skin in contrast to non-lesional skin of plaque psoriasis patients and in synovial tissue of psoriatic arthritis patients. The frequency of IL-17-producing cells was also significantly higher in the subchondral bone marrow of facet joints from patients with ankylosing spondylitis. Secukinumab selectively binds to and neutralizes IL-17A, preventing its interaction with the IL-17 receptors expressed on keratinocytes, fibroblast-like synoviocytes, endothelial cells, chondrocytes and osteoblasts. As a result, secukinumab inhibits the release of proinflammatory cytokines, chemokines and mediators of tissue damage and reduces IL-17A-mediated contributions to autoimmune and inflammatory diseases. Clinically relevant levels of secukinumab reach the skin and reduce local inflammatory markers. As a direct consequence treatment with secukinumab reduces erythema, induration and desquamation present in plaque psoriasis lesions.
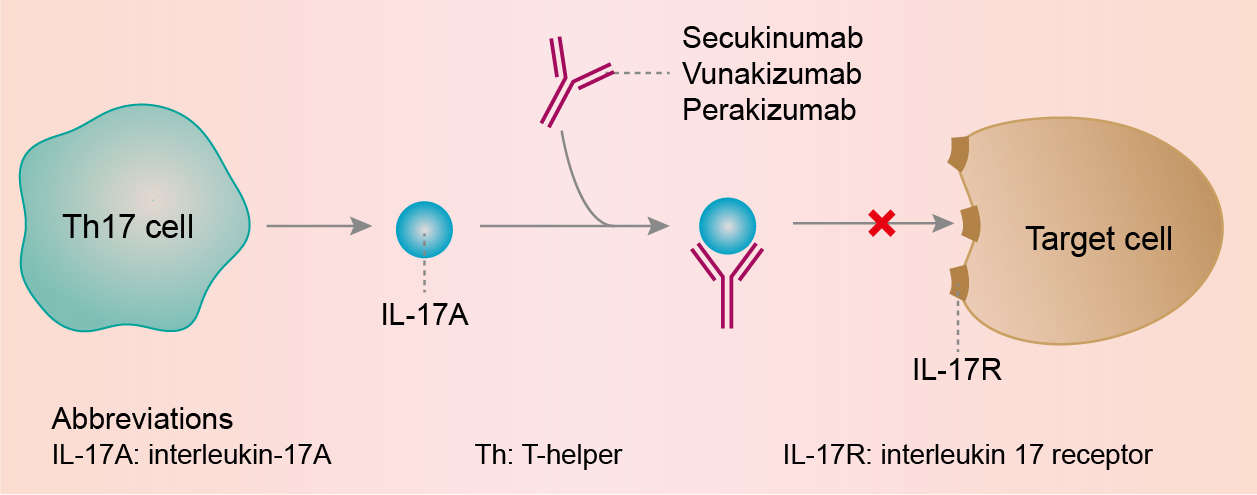 Fig.1 Mechanism of action of Secukinumab
Fig.1 Mechanism of action of Secukinumab
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

