Vedolizumab Overview
Introduction of Vedolizumab
Vedolizumab is a recombinant humanized IgG1 monoclonal antibody directed against the human lymphocyte α4β7 integrin, a key mediator of gastrointestinal inflammation. It is used in the treatment of moderate to severe active ulcerative colitis and Crohn's disease for patients who have had an inadequate response with, lost response to, or were intolerant to inhibitors of tumor necrosis factor-alpha (TNF-alpha) or other conventional therapies. By blocking its primary target, α4β7 integrin, vedolizumab reduces inflammation in the gut. Vedolizumab is administered by IV infusion over a period of 30 minutes; after the first dose, it is given again at two and six weeks and then every 8 weeks thereafter.
Mechanism of Action of Vedolizumab
The inflammatory bowel diseases (IBDs) are chronic inflammatory disorders of the GI tract consisting primarily of two subtypes, ulcerative colitis (UC) and Crohn’s disease (CD). CD is characterized by transmural inflammation of any portion of the GI tract from mouth to anus, whereas UC is limited to the colon and rectum with inflammation typically restricted to the mucosa, although inflammatory involvement of the submucosa may occur in severe cases. Both CD and UC are characterized by an influx of inflammatory cells, especially leukocytes into gut mucosal tissue. An important leukocyte involved in the pathogenesis of both CD and UC is the α4β7-integrin-expressing T cell. Integrins, which consist of an α- and β-chain that together form a heterodimer, bind to ligands on endothelial cells, allowing leukocytes to firmly adhere to endothelial surfaces.
Extravasation of leukocytes from the blood into stromal tissues of gut mucosal tissue is a complex process involving a coordinated sequence of events between leukocytes and vascular endothelial cells. Several steps – tethering/rolling, activation, adhesion and extravasation/migration occur allowing immune cells to enter stromal tissues. Initially, leukocytes tether to the vascular endothelium through multiple transient interactions between selectins on leukocytes such as PSGL-1 and their ligands on the endothelial surface (P-selectin and E-selectin). This process decreases the speed of leukocytes to facilitate rolling along the endothelial surface. The slower speed of these leukocytes permits interactions to occur between integrins on the surface of the leukocyte and their ligands on the endothelium. Slowing leukocytes also allows chemokines from inflamed tissue to activate them resulting in leukocyte polarization, while also enhancing the binding affinity of integrins. When activated, these cells preferentially adhere to endothelial surfaces within the GI tract as well as the associated lymphoid tissues. Upon activation, α4β7-integrin-expressing T cells bind to their ligand MAdCAM-1 and leukocytes are primed for extravasation. Leukocytes subsequently cross the endothelium and enter the mucosa through a paracellular route.
Vedolizumab was designed specifcally targets α4β7, so that preventing the α4β7/MAdCAM-1 interaction, hence inhibiting leukocyte migration into gut mucosa. Commendably, based on studies performed by Millennium Pharmaceuticals (MA, USA), vedolizumab does not affect levels of T cells in the cerebrospinal fluid of healthy volunteers after a single dose nor does it inhibit immune surveillance of the CNS in nonhuman primates.
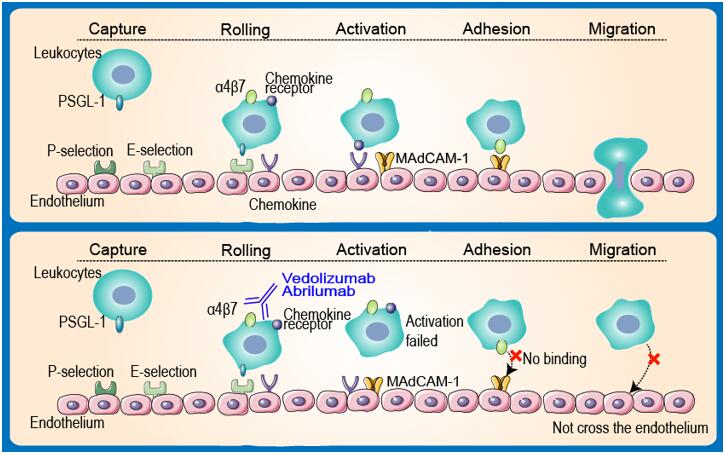 Figure 1 Mechanism of Action of Vedolizumab
Figure 1 Mechanism of Action of Vedolizumab
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.



