Visilizumab Overview
Introduction of Visilizumab
Visilizumab is a humanized and non-Fc receptor (FcR)-binding IgG2 monoclonal antibody (MoAb) directed against CD3 with potential immunosuppressive activity. Visilizumab binds to invariant CD3 epsilon, one of the non-covalently-associated subunits of T-cell receptors (TCRs) on activated T cells. Firstly, it is being investigated for use as an immunosuppressive drug in patients with ulcerative colitis and Crohn's disease. At present, the potential therapeutic effect of visilizumab has been examined in several immune system–related disorders including ulcerative colitis, Crohn's disease, renal allograft, and acute graft-versus-host disease. Visilizumab has also been radiolabelled with technetium-99m for imaging T cells.
Mechanism of Action of Visilizumab
Acute graft-versus-host disease (GVHD) is mediated by donor T cells and remains a major barrier to successful hematopoietic cell transplantation despite prophylaxis using the best currently available drug combinations. Transplantation tolerance can be facilitated by activation-induced apoptosis of peripheral T cells triggered by specific antigens. Simultaneously, more than 95% of circulating human peripheral T-cells and activated T-cells in inflamed tissues express CD3 antigen. Visilizumab (HuM291, Nuvion, Plymouth, MN) is a novel non–FcR-binding anti-CD3 monoclonal antibody (mAb) directed against the invariant CD3ε chain of the TCR. Its human IgG2 isotype confers the longest in vivo half-life among all human IgGs and reduces its ability to activate human complement or to interact with type I and III FcRs. Engineered mutations within the IgG2 Fc at amino acid residues 234 and 237 (Val→Ala) confer the inability to bind type II FcRs. Unlike the prototypic murine anti-CD3 mAbs, which induce T-cell activation by FcR binding and recruitment of antigen-presenting cells, non–FcR-binding anti-CD3 mAbs do not activate resting T cells and therefore induce less toxicity from cytokine release in vivo. Visilizumab dissociates quickly from and causes minimal internalization of the TCR. Durable expression of the TCR allows sustained signaling by visilizumab leading to apoptosis.
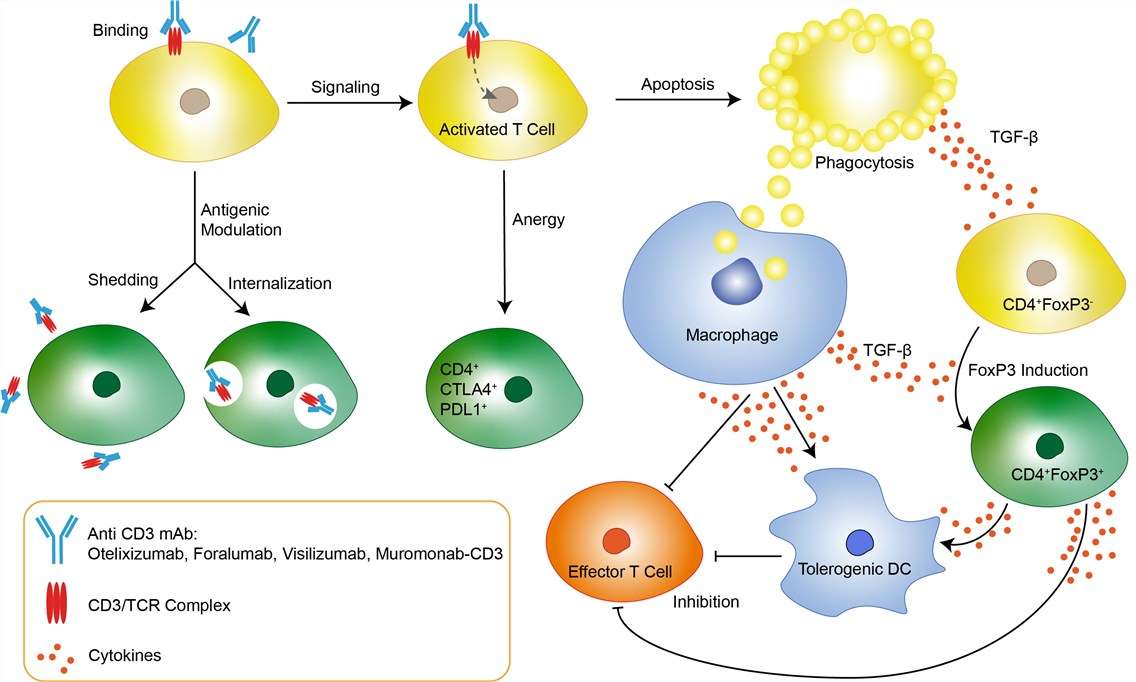 Fig.1 Mechanism of action of Visilizumab
Fig.1 Mechanism of action of Visilizumab
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

