Bevacizumab Overview
Introduction of Bevacizumab
Bevacizumab is a recombinant humanized monoclonal antibody (mAb) that blocks angiogenesis by inhibiting vascular endothelial growth factor A (VEGF-A). Bevacizumab was originally derived from a mouse mAb generated from mice immunized with the 165-residue- form of recombinant human vascular endothelial growth factor (VEGF). It was humanized by retaining the binding region and replacing the rest with a human full light chain and a human truncated IgG1 heavy chain, with some other substitutions. The resulting plasmid was transfected into Chinese Hamster Ovary (CHO) cells which are grown in industrial fermentation systems. As the first available angiogenesis inhibitor in the United States, bevacizumab was approved for medical use by the Food and Drug Administration (FDA) in 2004. Bevacizumab has been successively approved for the treatment of colorectal cancer, Lung cancer, breast cancer, renal cancers, brain cancers, and ovarian cancer. Bevacizumab was also found to be effective against eye diseases such as age-related macular degeneration.
Mechanism of Action of Bevacizumab
Angiogenesis is a very complex and highly regulated process by which tumors larger than 1 mm are thought to develop new vasculature. This constitutes an essential feature of cancer, considering that in order for cancer cells to proliferate, a continuous supply of oxygen is needed. Without neovascularization, tumor growth is arrested. The mechanisms through which tumors acquire neovascularity are (a) endothelial ‘‘sprouting,’’ wherein normal luminal endothelial cells migrate through the basement membrane into the underlying extracellular matrix and to the tumor, developing a morphology that resembles plant sprouts; (b) co-option of pre-existing vasculature, wherein tumor cells grow around blood vessels; (c) vasculogenic mimicry, wherein aggressive tumor cells develop microvascular channels; and (d) tumor neovascularization per se, wherein the release of proangiogenic factors, such as VEGF, fibroblast growth factor, and platelet-derived growth factor (PDGF), by endothelial, stromal, and tumor cells, causes endothelial activation, vessel growth, and tumor expansion. The VEGF family (VEGF-A, VEGF-B, VEGF-C, VEGF-D, VEGF-E, and placenta growth factor 1 and 2) and their corresponding VEGF tyrosine kinase receptors (VEGFR-1, VEGFR-2, and VEGFR-3) have emerged as promising targets because they have an important role in angiogenesis and in the growth of tumors. This is especially true of serum VEGF-A, the levels of which are higher in tumor tissue than in normal tissue, specifically in cancers of the breast, lung, colon, uterus, and ovary, which has been associated with poor prognosis. When VEGF-A binds to its receptor (VEGFR-2 and less specifically to VEGFR1), it activates a signaling cascade mediated by MAP kinase and PI3K/Akt/mTOR that results in the development of angiogenesis, increased vascular permeability, and lymphangiogenesis. Bevacizumab binds to VEGF-A, and thereby inhibits the binding of VEGF-A to its receptors on the surface of endothelial cells. Neutralizing the biological activity of VEGF regresses the vascularization of tumors, normalizes remaining tumour vasculature, and inhibits the formation of new tumour vasculature, thereby inhibiting tumour growth.
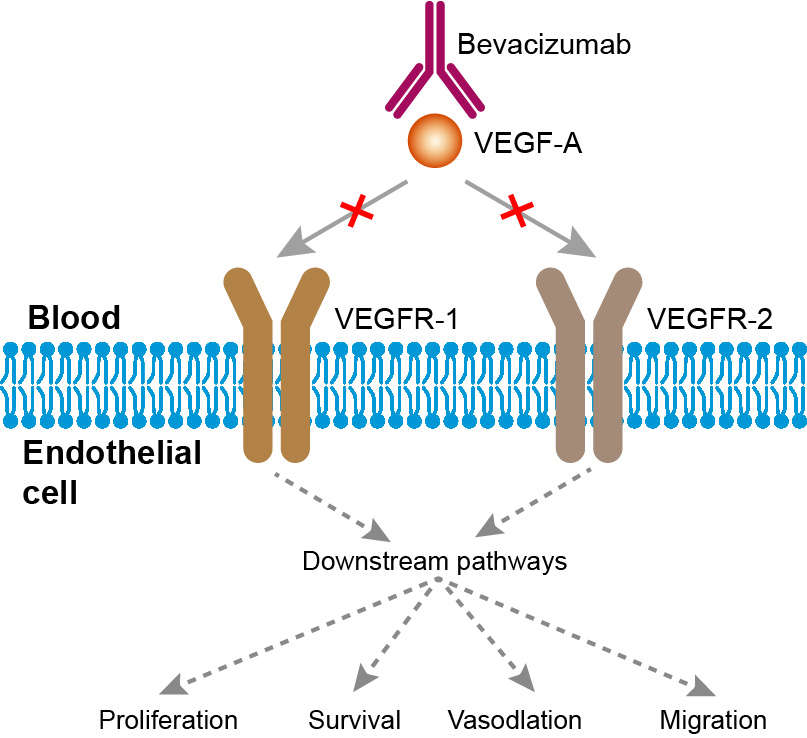 Fig.1 Mechanism of action of Bevacizumab
Fig.1 Mechanism of action of Bevacizumab
What We Provide
Therapeutic Antibody
Bevacizumab
We provide high-quality Bevacizumab for use in WB, FC, IP, ELISA, Neut, FuncS, IF and most other immunological methods. For lab research use only, not for diagnostic, therapeutic or any in vivo human use.
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.




