Trastuzumab Overview
Introduction of Trastuzumab
Trastuzumab is a recombinant IgG1 kappa, humanized monoclonal antibody (mAb) that selectively binds with high affinity to the extracellular domain of the human epidermal growth factor receptor (HER2) protein. It is produced in Chinese hamster ovary (CHO) cell culture. It is for the treatment of early stage HER2-positive breast cancer, or metastatic breast cancer that substantially overexpress HER2. It may be used by itself or together with other chemotherapy medication. Trastuzumab is given by slow injection into a vein and injection just under the skin. Trastuzumab was approved for medical use in the United States in 1998. It is on the World Health Organization's List of Essential Medicines, the most effective and safe medicines needed in a health system.
Mechanism of Action of Trastuzumab
The HER (human epidermal growth factor receptor) protein, binds to human epidermal growth factor, and stimulates cell proliferation. In some cancers, notably certain types of breast cancer, HER2 is over-expressed and causes cancer cells to reproduce uncontrollably. HER2 extends across the cell membrane, and carries signals from outside the cell to the inside. Signaling compounds called mitogens (specifically EGF in this case) arrive at the cell membrane, and bind to the extracellular domain of the HER family of receptors. Those bound proteins then link (dimerize), activating the receptor. HER2 sends a signal from its intracellular domain, activating several different biochemical pathways. These include the PI3K/Akt pathway and the MAPK pathway. Signals on these pathways promote cell proliferation and the growth of blood vessels to nourish the tumor (angiogenesis). Because it was recognized that constitutive HER2 signaling can drive breast tumor development and progression, strategies were developed to therapeutically target HER2 activity. The first successful approach began with the development of a mouse mAb called 4D5, which targets an extracellular epitope of the HER2 protein. HER2-specific monoclonal antibody 4D5 blocked phosphorylation of HER2 and suppressed growth of HER2-positive breast cancer cell lines and xenografts. In order to translate 4D5 clinically, a derivative that includes the antigen binding loops of 4D5 and the human variable region and immunoglobulin G1 constant domains was constructed. The recombinant humanized anti-HER2 antibody that resulted from this effort is called trastuzumab. Trastuzumab achieved significant regression of human HER2-overexpressing tumor xenografts as monotherapy or in combination with cytotoxic chemotherapeutic agents. The mechanisms underlying the anticancer activity of trastuzumab are not completely known, but several have been proposed. Some studies have suggested that trastuzumab downregulates total levels of HER2 on the cell surface, which may ultimately reduce downstream PI3K and MAPK signaling. Trastuzumab has been shown to block cleavage of the extracellular domain of HER2, thus, preventing formation of the constitutively active membrane-bound 95-kiloDalton HER2 protein called p95 HER2. Trastuzumab may also selectively inhibit HER2-HER3 heterodimerization, reducing PI3K signaling. Induction of cell cycle arrest by trastuzumab is mediated by p27kip1 and inhibition of cdk2 activity. Furthermore, trastuzumab appears to reduce angiogenesis, which may result in increased permeability of blood vessels, potentially increasing delivery of drugs to the tumor. Finally, HER2-positive cells that become “coated” in trastuzumab will be recognized by specific immune cells, causing antibody-dependent cellular cytotoxicity (ADCC), or lysis of the antibody-bound cells.
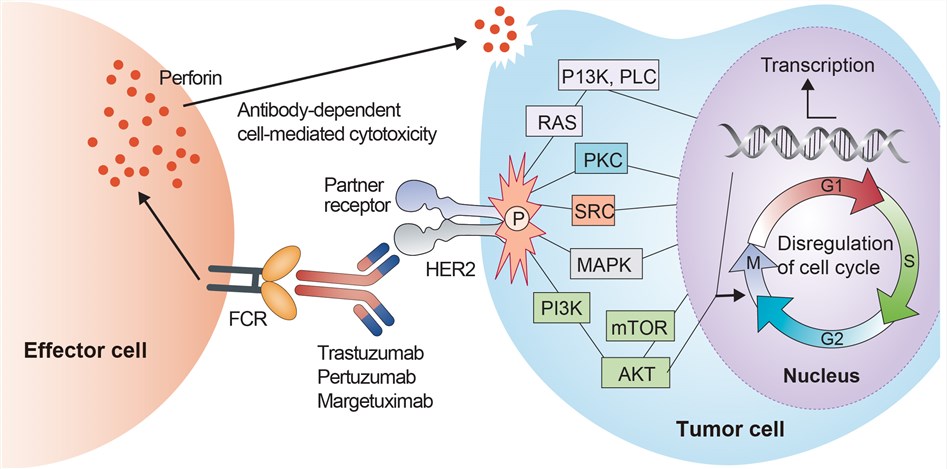 Fig.1 Mechanism of action of Trastuzumab
Fig.1 Mechanism of action of Trastuzumab
What We Provide
Therapeutic Antibody
Trastuzumab
We provide high-quality Trastuzumab for use in WB, FC, IP, ELISA, Neut, FuncS, IF and most other immunological methods. For lab research use only, not for diagnostic, therapeutic or any in vivo human use.
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.




