Endometrial Cancer Overview
About Endometrial Cancer
Endometrial cancer is the most common gynecologic malignancy. It is the fourth most common cancer in women after breast, lung, and colorectal cancers. Endometrial cancer is generally classified into two types. Type I is the most common form, representing more than 70% of cases. Type I tumors are associated with unopposed estrogen stimulation and are known as endometrioid adenocarcinoma. These tumors are generally low grade. Type II tumors are more likely to be high grade and of papillary serous or clear cell histologic type. They carry a poor prognosis and have a high risk of relapse and metastasis. Type II accounts for only 10% of endometrial cancers, but it is associated with 40% of related deaths. Familial tumors are commonly found in association with Lynch syndrome (hereditary nonpolyposis colorectal cancer). Genetic disease represents 10% of cases of endometrial cancer.
Overview of endometrial cancer pathways
Main Signaling Pathways in endometrial Cancer
Diagnosis of Endometrial Cancer
Most guidelines recommend either transvaginal ultrasonography or endometrial biopsy as the initial study for the evaluation of endometrial cancer. The American College of Radiology Appropriateness Criteria include tables outlining the preferred imaging studies for the evaluation of abnormal vaginal bleeding, including postmenopausal bleeding, and the pretreatment evaluation and follow-up of endometrial cancer. The type of initial study depends on the availability of options and their level of invasiveness, and patient and physician preference. Transvaginal ultrasonography is often the initial diagnostic study of choice when evaluating for endometrial cancer because of its availability, cost-effectiveness, and high sensitivity. Transvaginal ultrasonography can be used to measure endometrial thickness. The definitive diagnosis of endometrial cancer requires an endometrial tissue sample. When an adequate sample is obtained, the newer Pipelle method has high diagnostic accuracy, with a positive predictive value of 81.7% and a negative predictive value of 99.1%. Saline infusion sonohysterography can also be used to evaluate the endometrial cavity. This study technique uses saline infused into the endometrial cavity, followed by ultrasonography to allow better visualization of structural changes, particularly when patients have focal irregularities such as polyps, submucosal fibroids, or endometrial hyperplasia. Hysteroscopy is commonly used to evaluate abnormal uterine bleeding and offers direct visualization of the endometrial cavity. Hysteroscopy can be performed in conjunction with a focal biopsy or curettage. Magnetic resonance imaging may be able to provide additional information on endometrial thickening or structural abnormalities such as fibroids or adenomyosis when transvaginal ultrasonography is not adequate and saline infusion sonohysterography is not tolerated.
Targeted Therapy for Endometrial Cancer
Among targeted therapies that have been studied in the treatment of endometrial cancer, PI3K/Akt/mTOR pathway inhibitors have received the most attention. The PI3K/AKT/mTOR pathway is most frequently activated in endometrial cancer and promotes cellular growth, metabolism, proliferation, survival, migration, apoptosis and angiogenesis. In addition, overall 93% of endometrioid endometrial cancers had mutations of this pathway, suggesting the potential application of PI3K/AKT/mTOR pathway inhibitors as a targeted therapy. Representative agents include mTOR inhibitors (ridaforolimus, everolimus, temsirolimus), PI3K inhibitors (BKM120, Pilaralisib, PF-04691502 and PF05212384), dual-PI3K/mTOR inhibitors (GDC-0980) and AKT inhibitors (MK-2206).
HER2 (ERBB2) is a member of the human epidermal growth factor receptor family of transmembrane tyrosine kinase receptors and is amplified in 17–33% of uterine carcinosarcomas, serous carcinomas and a subset of high-grade endometrioid endometrial cancer. The activation of HER2 induces signal transduction through the MAPK- and PI3K signaling pathways and leads to the induction of cancer cell survival, proliferation, angiogenesis and metastasis. The objective response rate of trastuzumab, an anti-HER2-humanized monoclonal antibody (mAb), as a single agent in the first-line treatment of HER2- overexpressing metastatic breast cancer was 26%, and the overall tumor response rate of trastuzumab in combination with chemotherapy for the treatment of HER2-positive advanced gastric or gastro-esophageal junction cancer was 47%. 20% of tumors in women with advanced or recurrent endometrial cancer who were treated in a front-line chemotherapy trial showed HER2 gene amplification and 20% were strongly immunohistochemically positive for HER2.
The epidermal growth factor receptor (EGFR) belongs to the ErbB family of receptor tyrosine kinases. Ligand-dependent EGFR activation transduces multiple signaling pathways, including the RAS pathway, MAPK pathway, PI3K/Akt pathway, mTOR pathway and the phospholipase C (PLC)/protein kinase C (PKC) signaling cascade. Canonical EGFR signaling is critical for several cellular functions including survival, proliferation, differentiation and motility. Erlotinib demonstrated a relatively high response rate of 12.5%, which lasted 2–36 months in 32 patients with recurrent or metastatic endometrial cancer; however, a molecular analysis did not identify EGFR mutations in responders or a correlation between the response and gene amplification. Then, a phase 2 trial was performed to evaluate the efficacy and safety of lapatinib, a tyrosine kinase inhibitor (TKI) of EGFR and HER2.
Folate receptor (FR) is associated with cancer development, progression and metastasis and is markedly overexpressed on the surface of 64% of endometrial cancers. Several lines of evidence suggest that the expression of FR increases as the disease stage advances.
MEK is a kinase in the MAPK signal transduction pathway for many growth factor receptors including EGFR, insulin-like growth factor (IGF)−1 receptor and platelet-derived growth factor receptor (PDGFR). The activation of MEK leads to the phosphorylation of ERK1 and 2 to promote cell survival, proliferation and differentiation. In a phase 2 evaluation of selumetinib, a selective MEK-1/2 inhibitor, in the treatment of recurrent or persistent endometrial cancer, 6% of the patients had an objective response.
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.


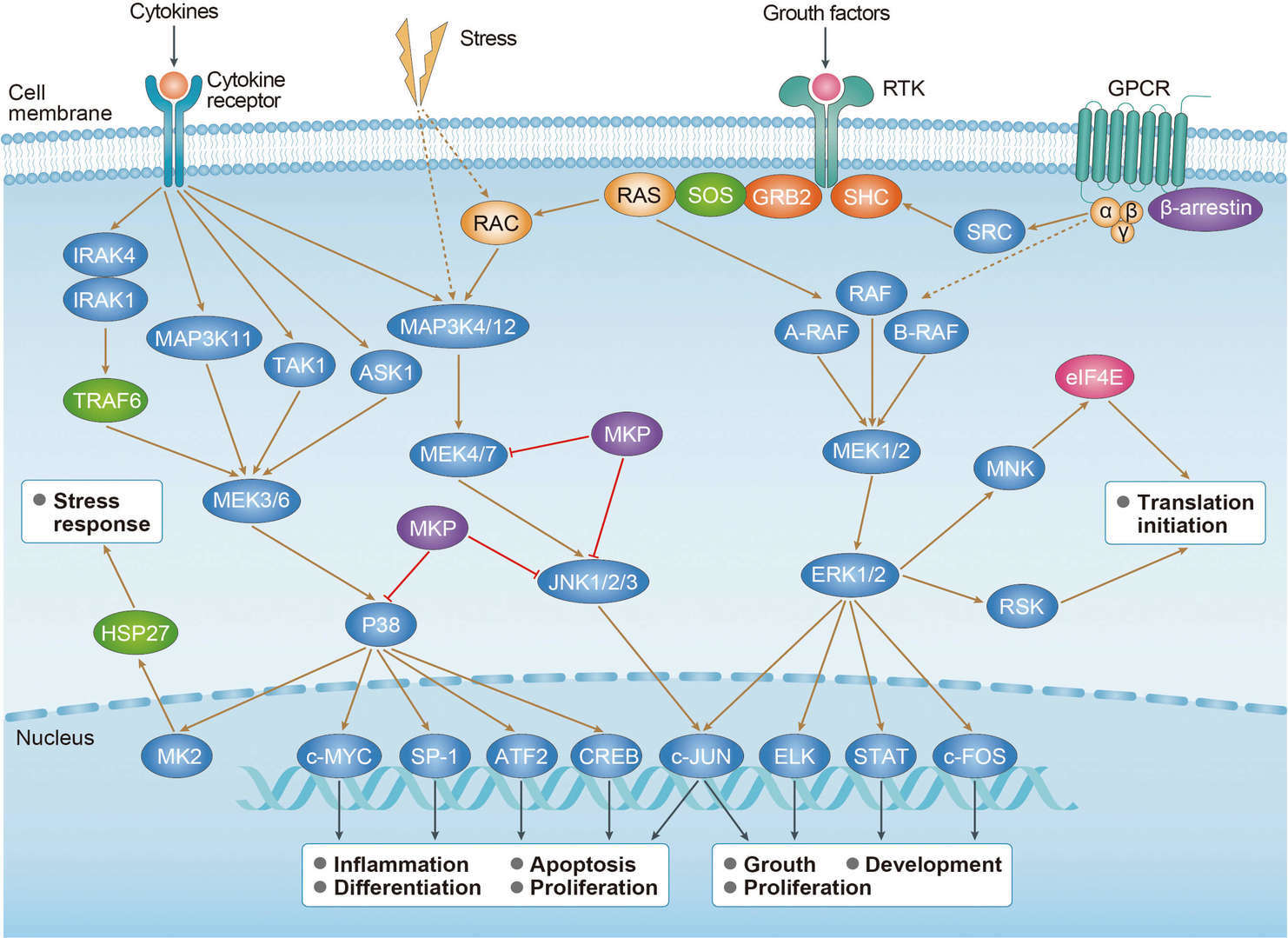 MAPK Signaling Pathway
MAPK Signaling Pathway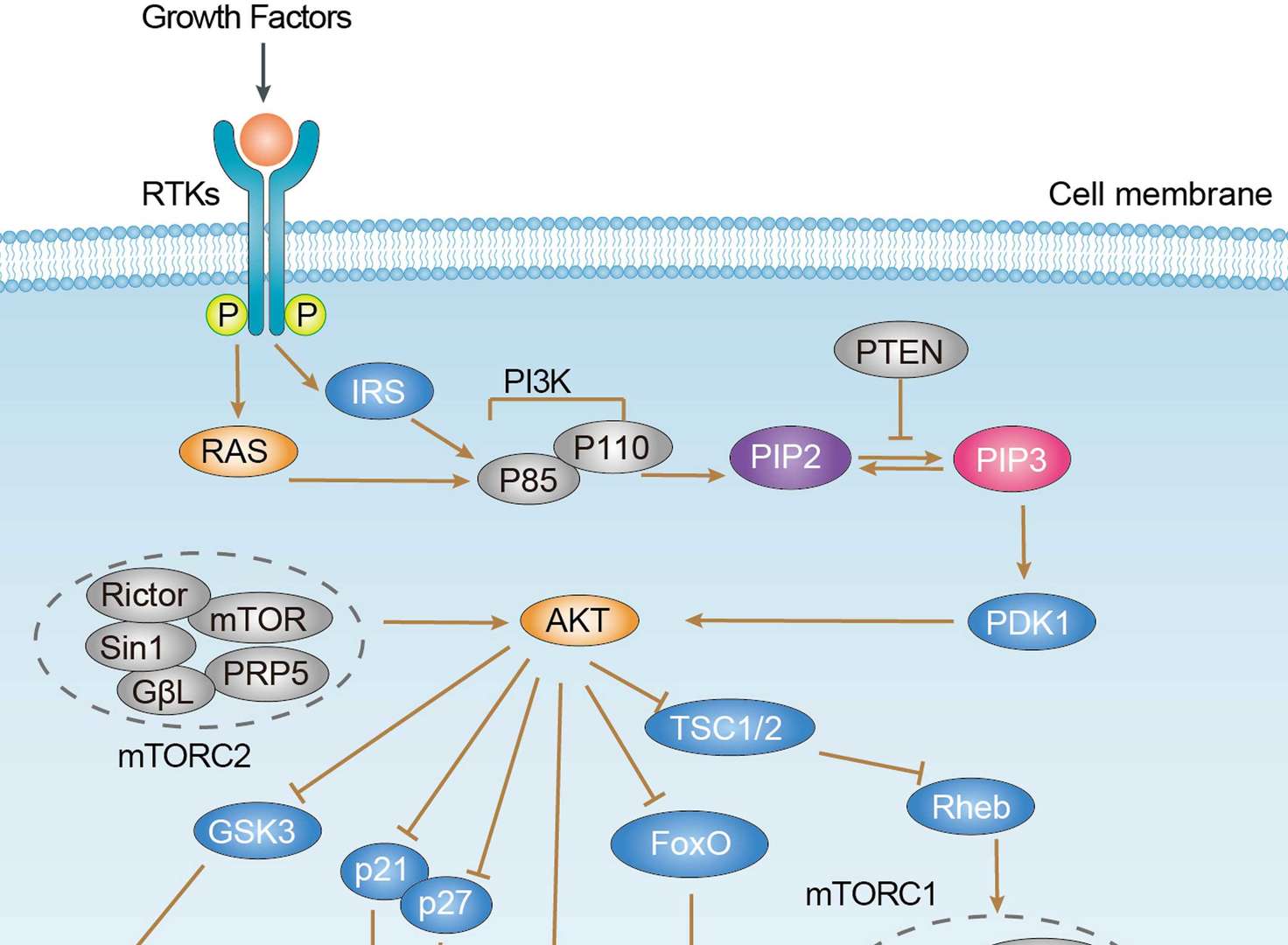 PI3K-Akt Signaling Pathway
PI3K-Akt Signaling Pathway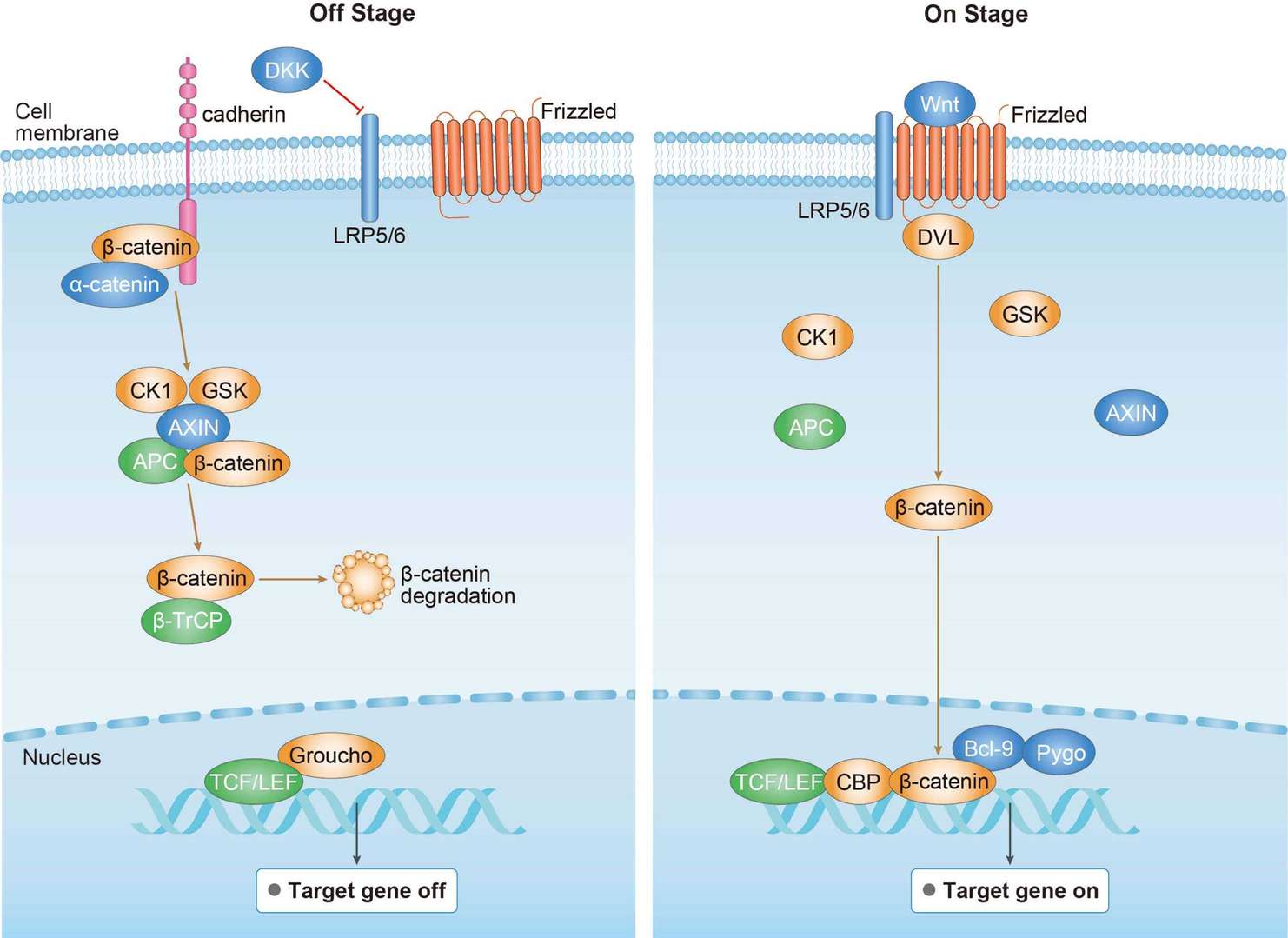 Wnt Pathway
Wnt Pathway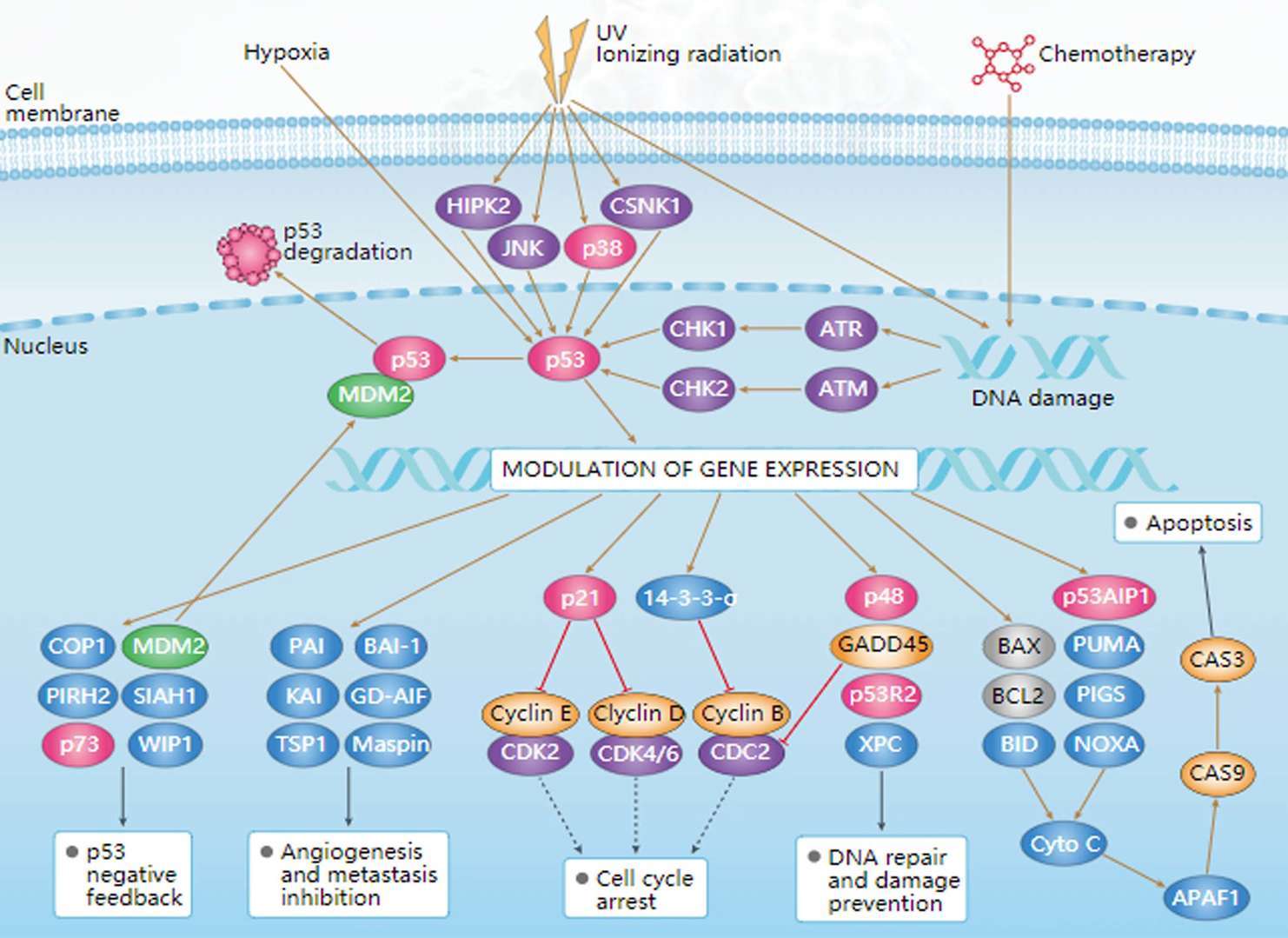 p53 Pathway
p53 Pathway
