Gastric Cancer Overview
About Gastric Cancer
Gastric cancer (GC) is one of the world's most common cancers. According to Lauren's histological classification gastric cancer is divided into two distinct histological groups-the intestinal and diffuse types. A more recent classification is based on mucin expression and distinguishes 4 types of gastric cancer: the gastric or foveolar type (G-type), the intestinal type (I-type), the gastric and intestinal mixed type (GI-type) and the neither gastric nor intestinal phenotypes (N-type). Risk factors include helicobacter pylori infection, gastric lymphoma, pernicious anemia, inherited cancer syndromes and family history. Tests that examine the gastric and esophagus are used to detect (find) and diagnose gastric cancer. Gastric cancers tend to develop slowly over many years. Before a true cancer develops, pre-cancerous changes often occur in the inner lining (mucosa) of the gastric. Early-stage gastric cancer rarely causes symptoms, and this is one of the reasons gastric cancer is so hard to detect early. Studies in the United States have not found that routine screening in people at average risk for gastric cancer is useful, because this disease is not that common. Six types of standard treatment are used for gastric cancer, such as surgery, chemotherapy, radiation therapy, chemoradiation, targeted therapy and immunotherapy.
Main Signaling Pathways in Gastric Cancer
Diagnosis of Gastric Cancer
Tests that examine the stomach and esophagus are used to detect (find) and diagnose gastric cancer. Common diagnostic procedures include six types. Physical exam and history: An exam of the body to check general signs of health, including checking for signs of disease, such as lumps. A history of the patient’s health habits and past illnesses and treatments will also be taken. Blood chemistry studies: A procedure in which a blood sample is checked to measure the amounts of certain substances released into the blood by organs and tissues in the body. Complete blood count (CBC): A procedure in which a sample of blood is drawn and checked. Upper endoscopy: A procedure to look inside the esophagus, stomach, and duodenum to check for abnormal areas. Barium swallow: A series of x-rays of the esophagus and stomach. The patient drinks a liquid that contains barium, the liquid coats the esophagus and stomach, and x-rays are taken. CT scan (CAT scan): A procedure that makes a series of detailed pictures of areas inside the body, taken from different angles. This procedure is also called computed tomography, computerized tomography, or computerized axial tomography. Biopsy: The removal of cells or tissues so they can be viewed under a microscope to check for signs of cancer. A biopsy of the stomach is usually done during the endoscopy. The sample of tissue may also be checked for Helicobacter pylori infection. When gastric cancer is found very early, there is a better chance of recovery. At later stages, gastric cancer can be treated but rarely can be cured.
Targeted Therapy for Gastric Cancer
Trastuzumab is a monoclonal antibody, a man-made version of a very specific immune system protein, which targets the HER2 protein. Giving trastuzumab with chemo can help some patients with advanced, HER2-positive stomach cancer live longer than giving chemo alone. This drug only works if the cancer cells have too much HER2, so samples of your tumor must be tested to look for HER2 before starting treatment. Ramucirumab, one standard therapy for unresectable GC, is a monoclonal antibody that binds to a receptor for VEGF which can help slow or stop the growth and spread of cancer. Cetuximab is an IgG1 monoclonal antibody that inhibits ligand binding to the EGFR and stimulates cell-mediated cytotoxicity. Nimotuzumab is a recombinant humanized monoclonal immunoglobulin G1 antibody that acts against human EGFR and blocks the binding of EGF and transforming growth factor-α to EGFR. This mechanism inhibits cancer-cell proliferation, angiogenesis, and induces apoptosis. Apatinib is a small-molecule tyrosine kinase inhibitor (TKI) that highly selectively binds to and strongly inhibits VEGFR2 and decreases the VEGF-mediated endothelial cell migration, proliferation, and tumor microvascular density. Regorafenib is an oral multikinase inhibitor, targeted angiogenic (VEGFR1, VEGFR2, and TIE2), stromal and oncogenic receptor tyrosine kinases. Immunotherapies are being verified as potential therapies. Pembrolizumab is a selective, humanized, high-affinity IgG4κ monoclonal antibody designed to bind to PD-1 and block interactions between PD-1 and its ligands. By blocking PD-1, this drug with manageable toxicity profile and effective antitumor activity boosts the immune response against cancer cells.
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.


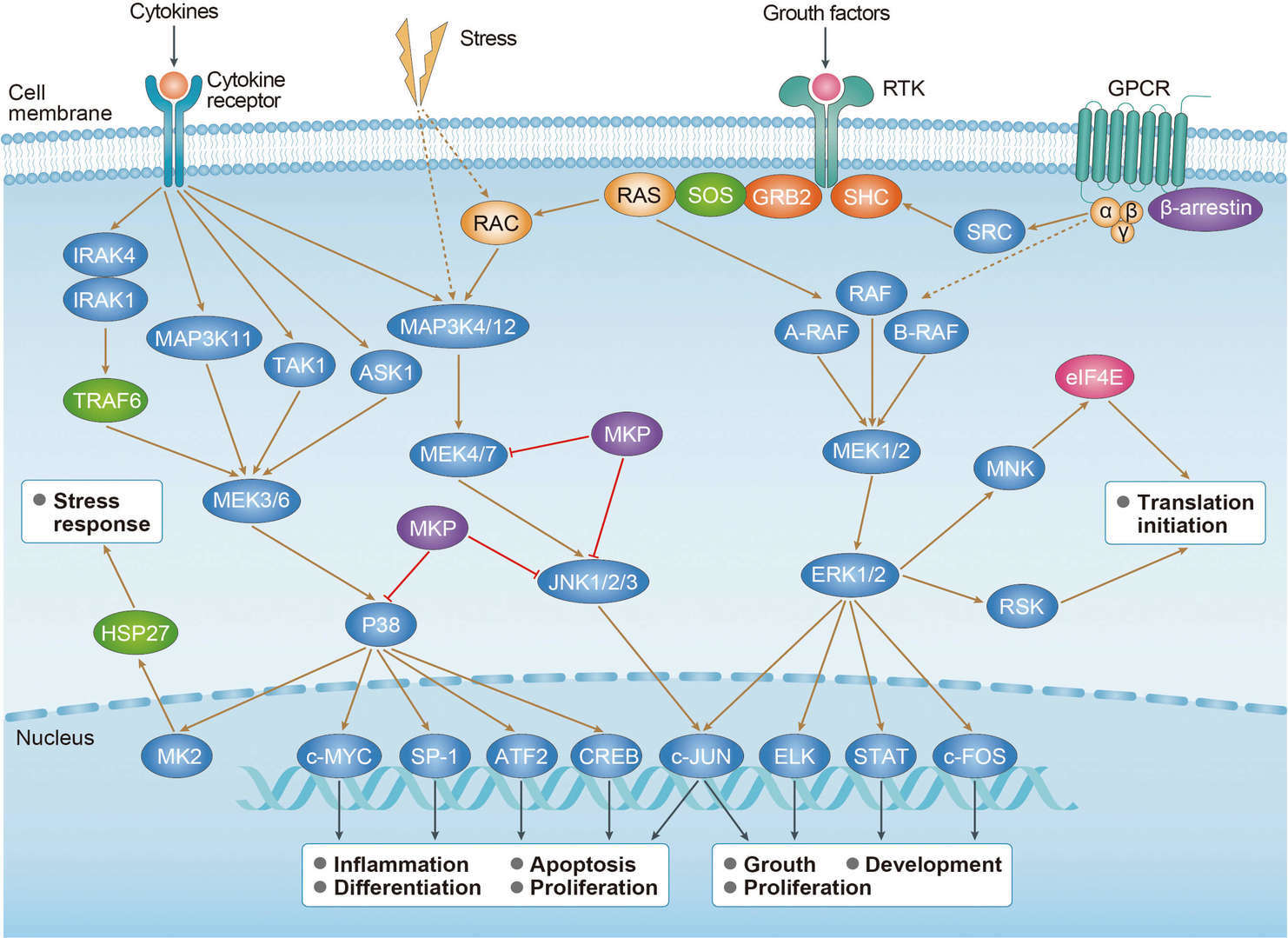 MAPK Pathway
MAPK Pathway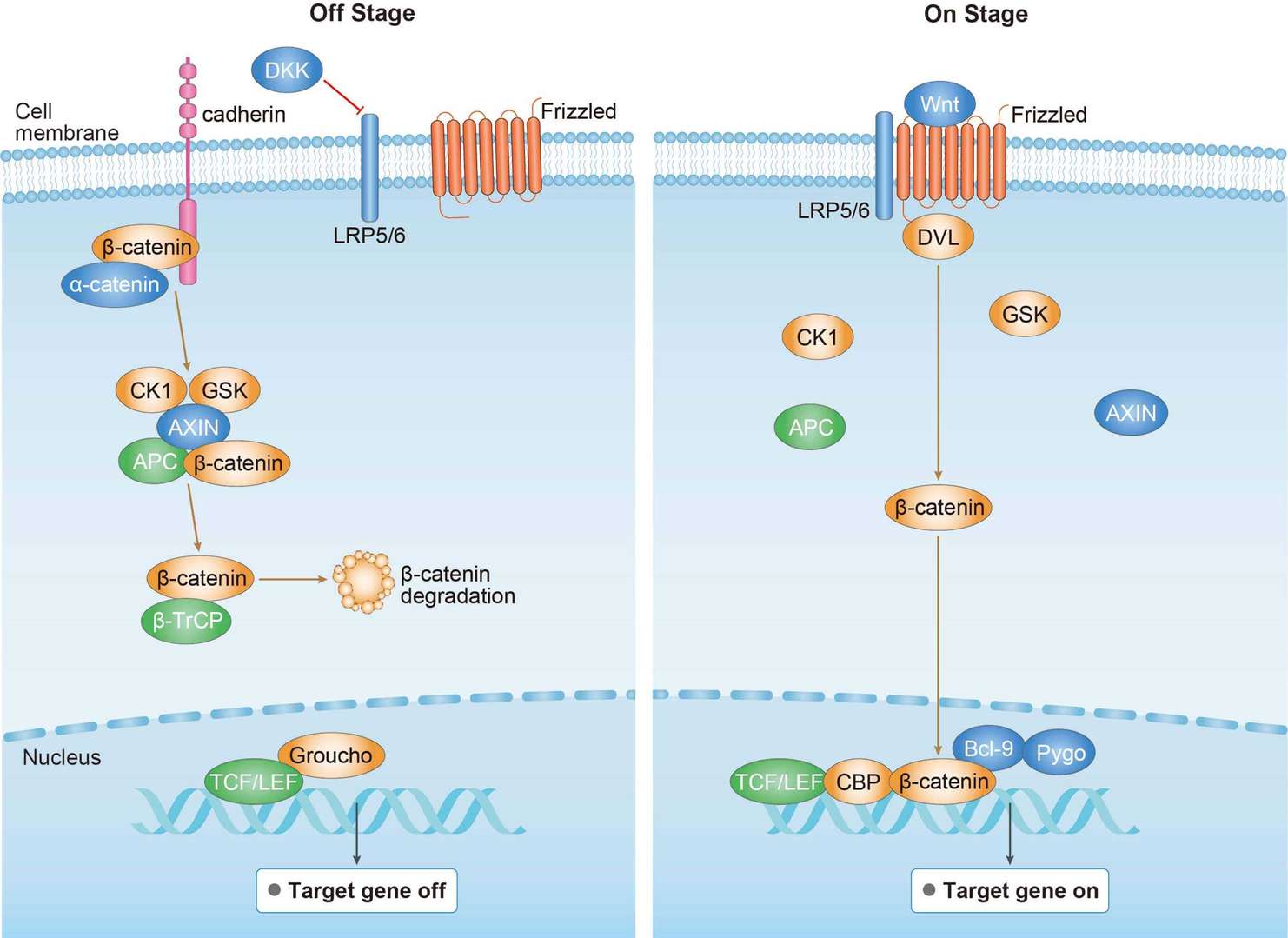 Wnt Pathway
Wnt Pathway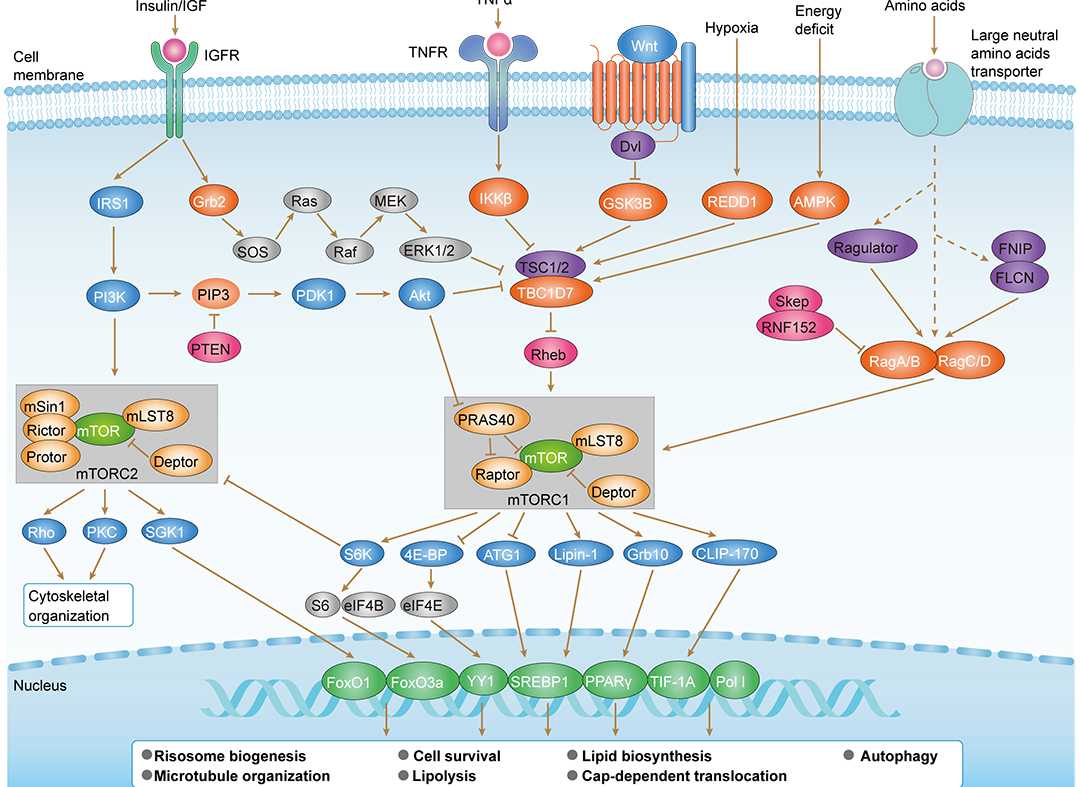 mTOR Pathway
mTOR Pathway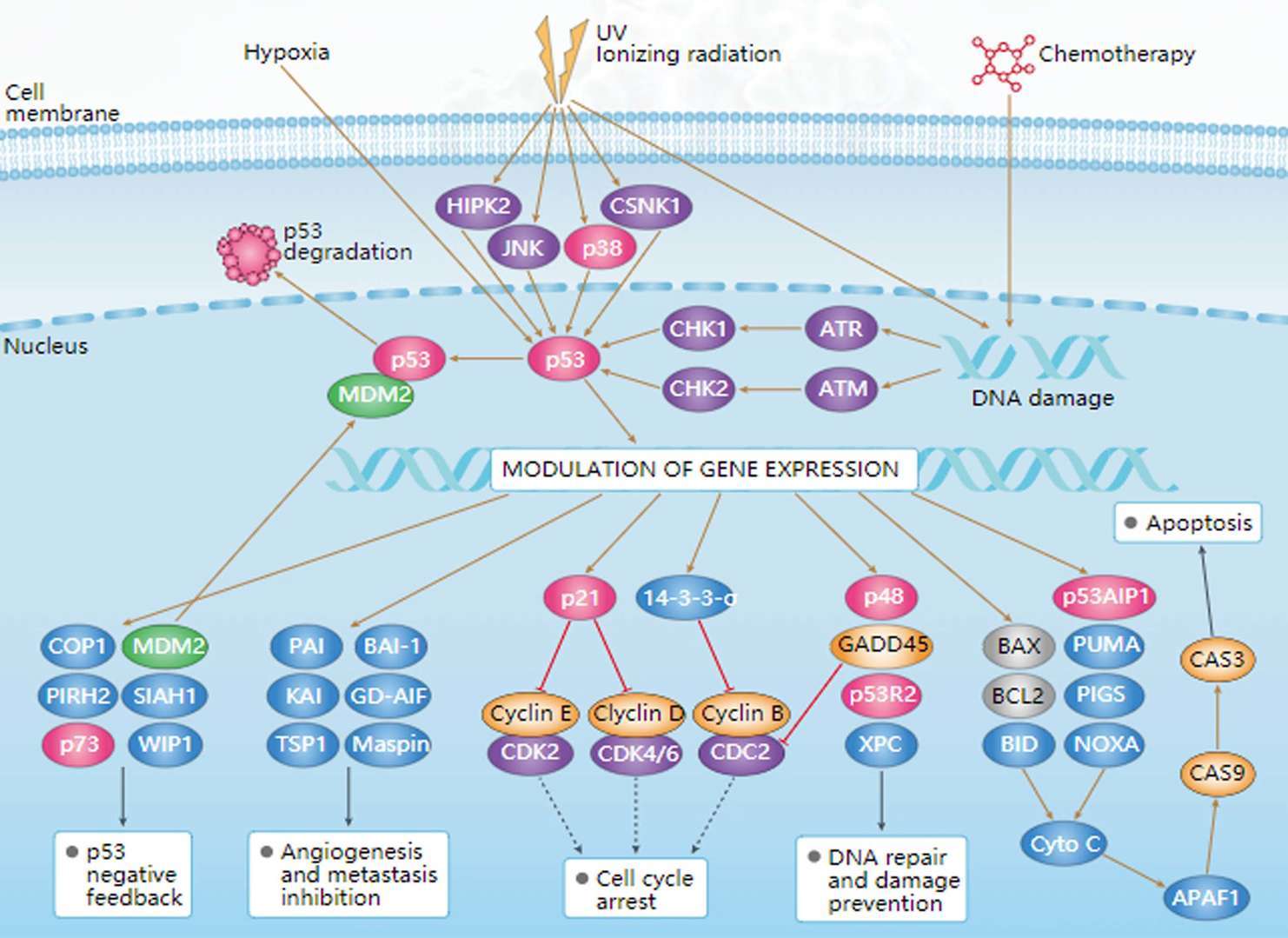 p53 Pathway
p53 Pathway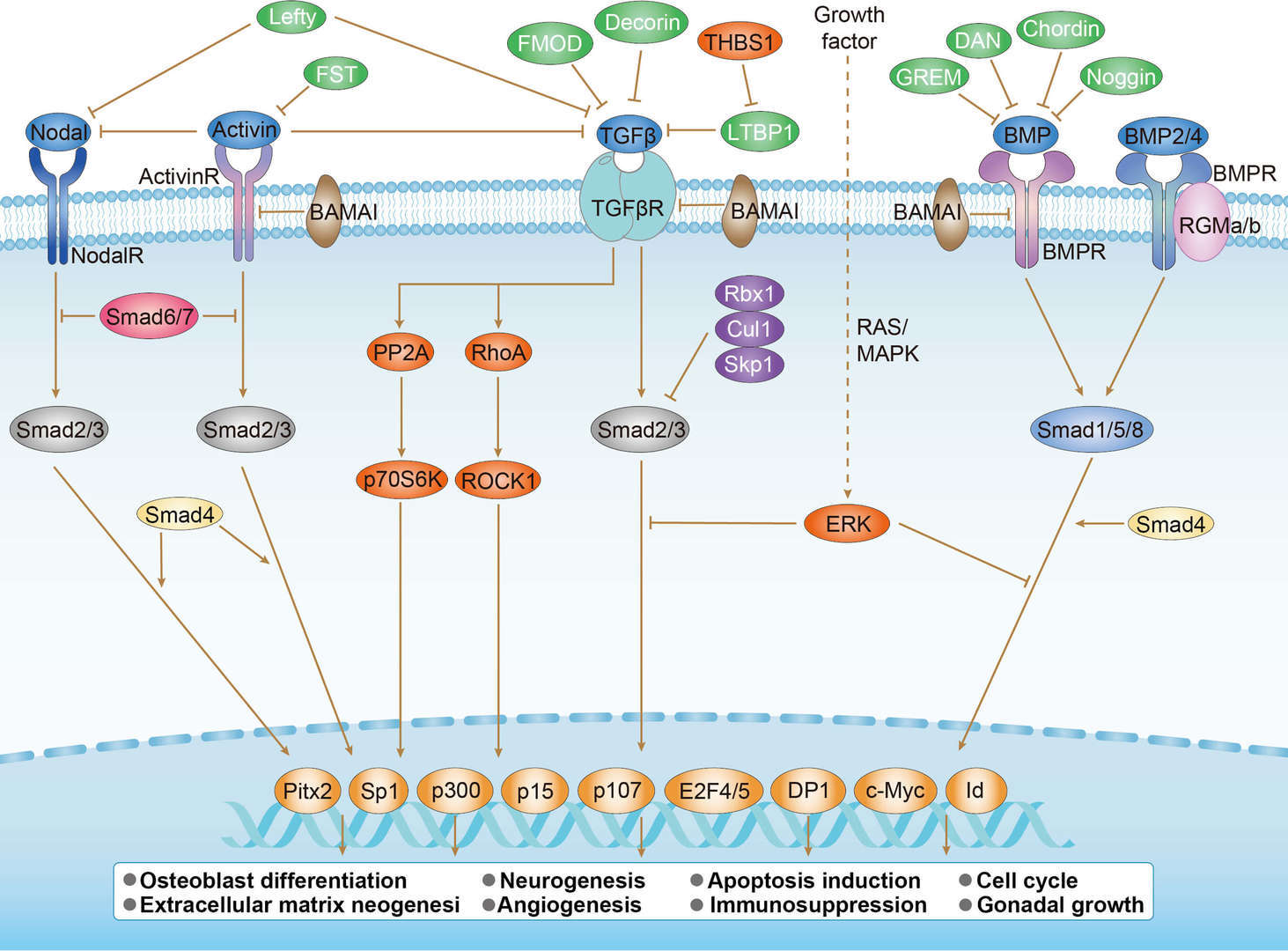 TGF-β Pathway
TGF-β Pathway